
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

Lipoic acid (LA), also known as α-lipoic acid, alpha-lipoic acid (ALA) and thioctic acid, is an organosulfur compound derived from caprylic acid (octanoic acid). ALA is made in animals normally, and is essential for aerobic metabolism. It is also manufactured and is available as a dietary supplement in some countries where it is marketed as an antioxidant, and is available as a pharmaceutical drug in other countries. Lipoate is the conjugate base of lipoic acid, and the most prevalent form of LA under physiological conditions. Only the (R)-(+)-enantiomer (RLA) exists in nature and is essential for aerobic metabolism because RLA is an essential cofactor of many enzyme complexes.
A polyamide is a polymer with repeating units linked by amide bonds.

Sodium amide, commonly called sodamide, is the inorganic compound with the formula NaNH2. It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent. NaNH2 conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. NaNH2 has been widely employed as a strong base in organic synthesis.

Cystinuria is an inherited autosomal recessive disease characterized by high concentrations of the amino acid cystine in the urine, leading to the formation of cystine stones in the kidneys, ureters, and bladder. It is a type of aminoaciduria. "Cystine", not "cysteine," is implicated in this disease; the former is a dimer of the latter.
The oxoglutarate dehydrogenase complex (OGDC) or α-ketoglutarate dehydrogenase complex is an enzyme complex, most commonly known for its role in the citric acid cycle.
Asparagusic acid is an organosulfur compound with the molecular formula C4H6O2S2 and systematically named 1,2-dithiolane-4-carboxylic acid. The molecule consists of a heterocyclic disulfide functional group (a 1,2-dithiolane) with a carboxylic acid side chain. It is found in asparagus and is believed to be the metabolic precursor to odorous sulfur compounds responsible for the distinctive smell of urine which has long been associated with eating asparagus.

Dihydrolipoyl transacetylase is an enzyme component of the multienzyme pyruvate dehydrogenase complex. The pyruvate dehydrogenase complex is responsible for the pyruvate decarboxylation step that links glycolysis to the citric acid cycle. This involves the transformation of pyruvate from glycolysis into acetyl-CoA which is then used in the citric acid cycle to carry out cellular respiration.
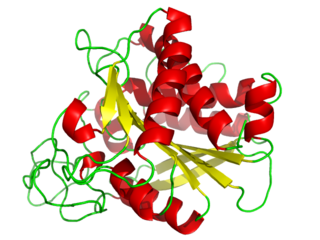
A carboxypeptidase is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed.
Oxidative decarboxylation is a decarboxylation reaction caused by oxidation. Most are accompanied by α- Ketoglutarate α- Decarboxylation caused by dehydrogenation of hydroxyl carboxylic acids such as carbonyl carboxylic acid, malic acid, isocitric acid, etc.
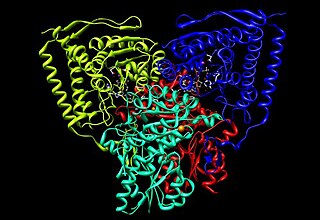
Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate.

Dihydrolipoamide dehydrogenase (DLD), also known as dihydrolipoyl dehydrogenase, mitochondrial, is an enzyme that in humans is encoded by the DLD gene. DLD is a flavoprotein enzyme that oxidizes dihydrolipoamide to lipoamide.
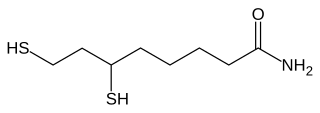
Dihydrolipoamide is a molecule oxidized by dihydrolipoyl dehydrogenase in order to produce lipoamide. Lipoamide is subsequently used as a cofactor for α-ketoglutarate dehydrogenase, the pyruvate dehydrogenase complex, and branched-chain α-ketoacid dehydrogenase complex.

Dihydrolipoic acid is an organic compound that is the reduced form of lipoic acid. This carboxylic acid features a pair of thiol groups, and therefore is a dithiol. It is optically active, but only the R-enantiomer is biochemically significant. The lipoic acid/dihydrolipoic acid pair participate in a variety of biochemical transformations.

Lipoyl synthase is an enzyme that belongs to the radical SAM (S-adenosyl methionine) family. Within the radical SAM superfamily, lipoyl synthase is in a sub-family of enzymes that catalyze sulfur insertion reactions. The enzymes in this subfamily differ from general radical SAM enzymes, as they contain two 4Fe-4S clusters. From these clusters, the enzymes obtain the sulfur groups that will be transferred onto the corresponding substrates. This particular enzyme participates in the final step of lipoic acid metabolism, transferring two sulfur atoms from its 4Fe-4S cluster onto the protein N6-(octanoyl)lysine through radical generation. This enzyme is usually localized to the mitochondria. Two organisms that have been extensively studied with regards to this enzyme are Escherichia coli and Mycobacterium tuberculosis. It is currently being studied in other organisms including yeast, plants, and humans.

2-Oxoisovalerate dehydrogenase subunit beta, mitochondrial is an enzyme that in humans is encoded by the BCKDHB gene.

Biochanin A is an O-methylated isoflavone. It is a natural organic compound in the class of phytochemicals known as flavonoids. Biochanin A can be found in red clover in soy, in alfalfa sprouts, in peanuts, in chickpea and in other legumes.

In molecular biology, the Cofactor transferase family is a family of protein domains that includes biotin protein ligases, lipoate-protein ligases A, octanoyl-(acyl carrier protein):protein N-octanoyltransferases, and lipoyl-protein:protein N-lipoyltransferases. The metabolism of the cofactors Biotin and lipoic acid share this family. They also share the target modification domain, and the sulfur insertion enzyme.
Biotin/lipoyl attachment domain has a conserved lysine residue that binds biotin or lipoic acid. Biotin plays a catalytic role in some carboxyl transfer reactions and is covalently attached, via an amide bond, to a lysine residue in enzymes requiring this coenzyme. Lipoamide acyltransferases have an essential cofactor, lipoic acid, which is covalently bound via an amide linkage to a lysine group. The lipoic acid cofactor is found in a variety of proteins.

Arachidonoyl serotonin is an endogenous lipid signaling molecule. It was first described in 1998 as being an inhibitor of fatty acid amide hydrolase (FAAH). In 2007, it was shown to have analgesic properties and to act as an antagonist of the TRPV1 receptor. In 2011, it was shown to be present in the ileum and jejunum of the gastrointestinal tract and modulate glucagon-like peptide-1 (GLP-1) secretion. In addition to this, in 2016, AA-5-HT was also found to affect the signaling mechanisms responsible for anxiety, by inhibiting dopamine release from the Basolateral amygdala following fear behavior. In 2017, AA-5-HT was tested in its effects on the sleep wake cycle, where it was found to affect the sleep homeostasis when used in conjunction with molecules and chemicals that affect wake-related neurotransmitters.

















