Related Research Articles

Mesalazine, also known as mesalamine or 5-aminosalicylic acid (5-ASA), is a medication used to treat inflammatory bowel disease, including ulcerative colitis and Crohn's disease. It is generally used for mildly to moderately severe disease. It is taken by mouth or rectally. The formulations which are taken by mouth appear to be similarly effective.

Capecitabine, sold under the brand name Xeloda among others, is a chemotherapy medication used to treat breast cancer, gastric cancer and colorectal cancer. For breast cancer it is often used together with docetaxel. It is taken by mouth.

Sorafenib, is a kinase inhibitor drug approved for the treatment of primary kidney cancer, advanced primary liver cancer, FLT3-ITD positive AML and radioactive iodine resistant advanced thyroid carcinoma.

Flupentixol (INN), also known as flupenthixol, marketed under brand names such as Depixol and Fluanxol is a typical antipsychotic drug of the thioxanthene class. It was introduced in 1965 by Lundbeck. In addition to single drug preparations, it is also available as flupentixol/melitracen—a combination product containing both melitracen and flupentixol. Flupentixol is not approved for use in the United States. It is, however, approved for use in the UK, Australia, Canada, Russian Federation, South Africa, New Zealand, Philippines and various other countries.
Propyphenazone/paracetamol/caffeine is an analgesic combination indicated for the management of headache. It contains the analgesics propyphenazone and paracetamol and the stimulant caffeine.
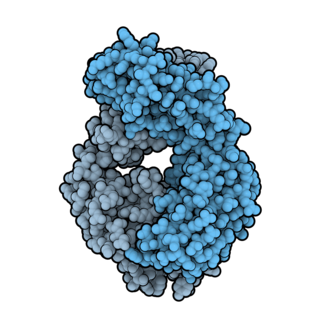
Ofatumumab, sold under the brand name Arzerra among others, is a fully human monoclonal antibody to CD20, which appears to inhibit early-stage B lymphocyte activation. It is FDA approved for treating chronic lymphocytic leukemia that is refractory to fludarabine and alemtuzumab and for the treatment of multiple sclerosis. It has also shown potential in treating follicular lymphoma, diffuse large B cell lymphoma, rheumatoid arthritis. Ofatumumab is the first marketing application for an antibody produced by Genmab, as well as the first human monoclonal antibody which targets the CD20 molecule that will be available for patients with refractory CLL.

Pazopanib, sold under the brand name Votrient, is an anti-cancer medication marketed worldwide by Novartis. It is a potent and selective multi-targeted receptor tyrosine kinase inhibitor that blocks tumour growth and inhibits angiogenesis. It has been approved for renal cell carcinoma and soft tissue sarcoma by numerous regulatory administrations worldwide.

Paricalcitol (chemically it is 19-nor-1,25-(OH)2-vitamin D2. Marketed by Abbott Laboratories under the trade name Zemplar) is a drug used for the prevention and treatment of secondary hyperparathyroidism (excessive secretion of parathyroid hormone) associated with chronic kidney failure. It is an analog of 1,25-dihydroxyergocalciferol, the active form of vitamin D2 (ergocalciferol).
Mirabegron, sold under the brand name Myrbetriq among others, is a medication used to treat overactive bladder. Its benefits are similar to antimuscarinic medication such as solifenacin or tolterodine. It is taken by mouth.
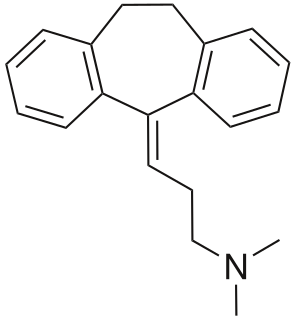
Amitriptyline/perphenazine is a formulation that contains the tricyclic antidepressant amitriptyline and the medium-potency typical (first-generation) antipsychotic, perphenazine. In the United States amitriptyline/perphenazine is marketed by Mylan Pharmaceuticals Inc. and Remedy Repack Inc.
References
- ↑ "Votrient (pazopanib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 27 January 2014.
- ↑ "VOTRIENT (pazopanib hydrochloride) tablet, film coated" (PDF). DailyMed. GlaxoSmithKline LLC. November 2013. Retrieved 27 January 2014.
- ↑ "Votrient : EPAR - Product Information" (PDF). European Medicines Agency. Glaxo Group Ltd. 23 January 2014. Retrieved 27 January 2014.
- ↑ "Votrient 200 mg and 400 mg film coated tablets - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. GlaxoSmithKline UK. 20 December 2013. Retrieved 27 January 2014.
- ↑ "PRODUCT INFORMATION VOTRIENT® TABLETS" (PDF). TGA eBusiness Services. GlaxoSmithKline Australia Pty Ltd. 25 March 2013. Retrieved 27 January 2014.