
A lithium-ion battery or Li-ion battery is a type of rechargeable battery composed of cells in which lithium ions move from the negative electrode through an electrolyte to the positive electrode during discharge and back when charging. Li-ion cells use an intercalated lithium compound as the material at the positive electrode and typically graphite at the negative electrode. Li-ion batteries have a high energy density, no memory effect and low self-discharge. Cells can be manufactured to either prioritize energy or power density. They can however be a safety hazard since they contain flammable electrolytes and if damaged or incorrectly charged can lead to explosions and fires.

A lithium polymer battery, or more correctly lithium-ion polymer battery, is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. High conductivity semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types and are used in applications where weight is a critical feature, such as mobile devices, radio-controlled aircraft and some electric vehicles.
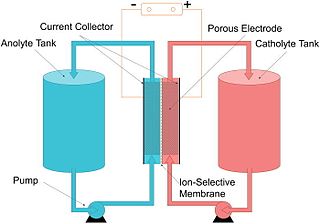
A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion exchange occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts.

Michael Stanley Whittingham is a British-American chemist. He is currently a professor of chemistry and director of both the Institute for Materials Research and the Materials Science and Engineering program at Binghamton University, State University of New York. He also serves as director of the Northeastern Center for Chemical Energy Storage (NECCES) of the U.S. Department of Energy at Binghamton. He was awarded the Nobel Prize in Chemistry in 2019 alongside Akira Yoshino and John B. Goodenough.
The lithium iron phosphate battery or LFP battery is a type of lithium-ion battery using lithium iron phosphate as the cathode material, and a graphitic carbon electrode with a metallic backing as the anode. The energy density of an LFP battery is lower than that of other common lithium ion battery types such as Nickel Manganese Cobalt (NMC) and Nickel Cobalt Aluminum (NCA), and also has a lower operating voltage;CATL's LFP batteries are currently at 125 Wh/kg, up to possibly 160 Wh/kg with improved packing technology, while BYD's LFP batteries are at 150 Wh/kg, compared to over 300 Wh/kg for the highest NMC batteries.
A polymer-based battery uses organic materials instead of bulk metals to form a battery. Currently accepted metal-based batteries pose many challenges due to limited resources, negative environmental impact, and the approaching limit of progress. Redox active polymers are attractive options for electrodes in batteries due to their synthetic availability, high-capacity, flexibility, light weight, low cost, and low toxicity. Recent studies have explored how to increase efficiency and reduce challenges to push polymeric active materials further towards practicality in batteries. Many types of polymers are being explored, including conductive, non-conductive, and radical polymers. Batteries with a combination of electrodes are easier to test and compare to current metal-based batteries, however batteries with both a polymer cathode and anode are also a current research focus. Polymer-based batteries, including metal/polymer electrode combinations, should be distinguished from metal-polymer batteries, such as a lithium polymer battery, which most often involve a polymeric electrolyte, as opposed to polymeric active materials.

Nanobatteries are fabricated batteries employing technology at the nanoscale, particles that measure less than 100 nanometers or 10−7 meters. These batteries may be nano in size or may use nanotechnology in a macro scale battery. Nanoscale batteries can be combined to function as a macrobattery such as within a nanopore battery.

Lithium iron phosphate (LFP) is an inorganic compound with the formula LiFePO
4. It is a gray, red-grey, brown or black solid that is insoluble in water. The material has attracted attention as a component of lithium iron phosphate batteries, a type of Li-ion battery. This battery chemistry is targeted for use in power tools, electric vehicles, solar energy installations and more recently large grid-scale energy storage.

A lithium-ion capacitor (LIC) is a hybrid type of capacitor classified as a type of supercapacitor. It is called a hybrid because the anode is the same as those used in lithium-ion batteries and the cathode is the same as those used in supercapacitors. Activated carbon is typically used as the cathode. The anode of the LIC consists of carbon material which is often pre-doped with lithium ions. This pre-doping process lowers the potential of the anode and allows a relatively high output voltage compared to other supercapacitors.
Nanoarchitectures for lithium-ion batteries are attempts to employ nanotechnology to improve the design of lithium-ion batteries. Research in lithium-ion batteries focuses on improving energy density, power density, safety, durability and cost.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
A potassium-ion battery or K-ion battery is a type of battery and analogue to lithium-ion batteries, using potassium ions for charge transfer instead of lithium ions. It was invented by the Iranian/American chemist Ali Eftekhari in 2004.
The sodium-ion battery (NIB or SIB) is a type of rechargeable battery analogous to the lithium-ion battery but using sodium ions (Na+) as the charge carriers. Its working principle and cell construction are almost identical with those of commercially widespread lithium-ion battery types, but sodium compounds are used instead of lithium compounds.
A lithium ion manganese oxide battery (LMO) is a lithium-ion cell that uses manganese dioxide, MnO
2, as the cathode material. They function through the same intercalation/de-intercalation mechanism as other commercialized secondary battery technologies, such as LiCoO
2. Cathodes based on manganese-oxide components are earth-abundant, inexpensive, non-toxic, and provide better thermal stability.
A lithium-ion flow battery is a flow battery that uses a form of lightweight lithium as its charge carrier. The flow battery stores energy separately from its system for discharging. The amount of energy it can store is determined by tank size; its power density is determined by the size of the reaction chamber.

Pseudocapacitance is the electrochemical storage of electricity in an electrochemical capacitor (Pseudocapacitor). This faradaic charge transfer originates by a very fast sequence of reversible faradaic redox, electrosorption or intercalation processes on the surface of suitable electrodes. Pseudocapacitance is accompanied by an electron charge-transfer between electrolyte and electrode coming from a de-solvated and adsorbed ion. One electron per charge unit is involved. The adsorbed ion has no chemical reaction with the atoms of the electrode since only a charge-transfer takes place.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and cost.
An organic radical battery (ORB) is a type of battery first developed in 2005. As of 2011, this type of battery was generally not available for the consumer, although their development at that time was considered to be approaching practical use. ORBs are potentially more environmentally friendly than conventional metal-based batteries, because they use organic radical polymers to provide electrical power instead of metals. ORBs are considered to be a high-power alternative to the Li-ion battery. Functional prototypes of the battery have been researched and developed by different research groups and corporations including the Japanese corporation NEC.
Magnesium batteries are batteries that utilize magnesium cations as the active charge transporting agent in solution and as the elemental anode of an electrochemical cell. Both non-rechargeable primary cell and rechargeable secondary cell chemistries have been investigated. Magnesium primary cell batteries have been commercialised and have found use as reserve and general use batteries.

This is a history of the lithium-ion battery.














