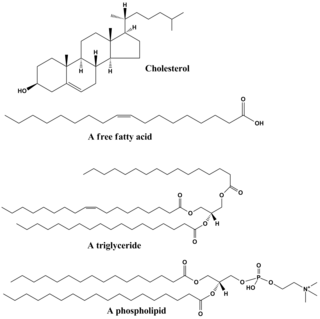
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue. Marine phospholipids typically have omega-3 fatty acids EPA and DHA integrated as part of the phospholipid molecule. The phosphate group can be modified with simple organic molecules such as choline, ethanolamine or serine.

A phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophilic substances. Acids trigger the release of bound calcium from cellular stores and the consequent increase in free cytosolic Ca2+, an essential step in calcium signaling to regulate intracellular processes. There are four major classes, termed A, B, C, and D, which are distinguished by the type of reaction which they catalyze:

Lecithin is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues which are amphiphilic – they attract both water and fatty substances, and are used for smoothing food textures, emulsifying, homogenizing liquid mixtures, and repelling sticking materials.

Phosphatidylcholines (PC) are a class of phospholipids that incorporate choline as a headgroup. They are a major component of biological membranes and can be easily obtained from a variety of readily available sources, such as egg yolk or soybeans, from which they are mechanically or chemically extracted using hexane. They are also a member of the lecithin group of yellow-brownish fatty substances occurring in animal and plant tissues. Dipalmitoylphosphatidylcholine (lecithin) is a major component of the pulmonary surfactant, and is often used in the lecithin–sphingomyelin ratio to calculate fetal lung maturity. While phosphatidylcholines are found in all plant and animal cells, they are absent in the membranes of most bacteria, including Escherichia coli. Purified phosphatidylcholine is produced commercially.
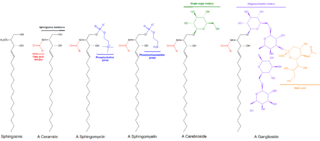
Sphingomyelin is a type of sphingolipid found in animal cell membranes, especially in the membranous myelin sheath that surrounds some nerve cell axons. It usually consists of phosphocholine and ceramide, or a phosphoethanolamine head group; therefore, sphingomyelins can also be classified as sphingophospholipids. In humans, SPH represents ~85% of all sphingolipids, and typically make up 10–20 mol % of plasma membrane lipids.

Phosphatidylinositol or inositol phospholipid is a biomolecule. It was initially called "inosite" when it was discovered by Léon Maquenne and Johann Joseph von Scherer in the late 19th century. It was discovered in bacteria but later also found in eukaryotes, and was found to be a signaling molecule.
Phosphatidic acids are anionic phospholipids important to cell signaling and direct activation of lipid-gated ion channels. Hydrolysis of phosphatidic acid gives rise to one molecule each of glycerol and phosphoric acid and two molecules of fatty acids. They constitute about 0.25% of phospholipids in the bilayer.
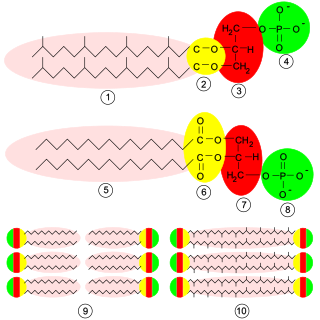
Glycerophospholipids or phosphoglycerides are glycerol-based phospholipids. They are the main component of biological membranes in eukaryotic cells. They are a type of lipid, of which its composition affects membrane structure and properties. Two major classes are known: those for bacteria and eukaryotes and a separate family for archaea.

Phosphatidylserine is a phospholipid and is a component of the cell membrane. It plays a key role in cell cycle signaling, specifically in relation to apoptosis. It is a key pathway for viruses to enter cells via apoptotic mimicry. Its exposure on the outer surface of a membrane marks the cell for destruction via apoptosis.
Phospholipase D (EC 3.1.4.4, lipophosphodiesterase II, lecithinase D, choline phosphatase, PLD; systematic name phosphatidylcholine phosphatidohydrolase) is an enzyme of the phospholipase superfamily that catalyses the following reaction
Lipid metabolism is the synthesis and degradation of lipids in cells, involving the breakdown and storage of fats for energy and the synthesis of structural and functional lipids, such as those involved in the construction of cell membranes. In animals, these fats are obtained from food and are synthesized by the liver. Lipogenesis is the process of synthesizing these fats. The majority of lipids found in the human body from ingesting food are triglycerides and cholesterol. Other types of lipids found in the body are fatty acids and membrane lipids. Lipid metabolism is often considered the digestion and absorption process of dietary fat; however, there are two sources of fats that organisms can use to obtain energy: from consumed dietary fats and from stored fat. Vertebrates use both sources of fat to produce energy for organs such as the heart to function. Since lipids are hydrophobic molecules, they need to be solubilized before their metabolism can begin. Lipid metabolism often begins with hydrolysis, which occurs with the help of various enzymes in the digestive system. Lipid metabolism also occurs in plants, though the processes differ in some ways when compared to animals. The second step after the hydrolysis is the absorption of the fatty acids into the epithelial cells of the intestinal wall. In the epithelial cells, fatty acids are packaged and transported to the rest of the body.

Phosphatidylethanolamine (PE) is a class of phospholipids found in biological membranes. They are synthesized by the addition of cytidine diphosphate-ethanolamine to diglycerides, releasing cytidine monophosphate. S-Adenosyl methionine can subsequently methylate the amine of phosphatidylethanolamines to yield phosphatidylcholines.

Phospholipase A1 (EC 3.1.1.32; systematic name: phosphatidylcholine 1-acylhydrolase) encoded by the PLA1A gene is a phospholipase enzyme which removes the 1-acyl group:
The enzyme lysophospholipase (EC 3.1.1.5) catalyzes the reaction
N-Acylphosphatidylethanolamines (NAPEs) are hormones released by the small intestine into the bloodstream when it processes fat. NAPEs travel to the hypothalamus in the brain and suppress appetite. This mechanism could be relevant for treating obesity.
N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) is an enzyme that catalyzes the release of N-acylethanolamine (NAE) from N-acyl-phosphatidylethanolamine (NAPE). This is a major part of the process that converts ordinary lipids into chemical signals like anandamide and oleoylethanolamine. In humans, the NAPE-PLD protein is encoded by the NAPEPLD gene.

In biochemistry, lipase refers to a class of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; however, these are usually treated separately from "conventional" lipases. Unlike esterases, which function in water, lipases "are activated only when adsorbed to an oil–water interface". Lipases perform essential roles in digestion, transport and processing of dietary lipids in most, if not all, organisms.

1-Lysophosphatidylcholines are a class of phospholipids that are intermediates in the metabolism of lipids. They result from the hydrolysis of an acyl group from the sn-1 position of phosphatidylcholine. They are also called 2-acyl-sn-glycero-3-phosphocholines. The synthesis of phosphatidylcholines with specific fatty acids occurs through the synthesis of 1-lysoPC. The formation of various other lipids generates 1-lysoPC as a by-product.
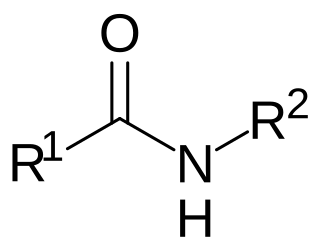
N-acyl amides are a general class of endogenous fatty acid compounds characterized by a fatty acyl group linked to a primary amine metabolite by an amide bond. Broadly speaking, N-acyl amides fall into several categories: amino acid conjugates, neurotransmitter conjugates, ethanolamine conjugates, and taurine conjugates. N-acyl amides have pleiotropic signaling functions in physiology, including in cardiovascular function, metabolic homeostasis, memory, cognition, pain, motor control and others. Initial attention focused on N-acyl amides present in mammalian organisms, however recently lipid signaling systems consisting of N-acyl amides have also been found to be present in invertebrates, such as Drosophila melanogaster. N-acyl amides play important roles in many biochemical pathways involved in a variety of physiological and pathological processes, as well as the metabolic enzymes, transporters, and receptors that regulate their signaling.














