
In biochemistry, a kinase is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. This transesterification produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group. These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis.
Inositol trisphosphate or inositol 1,4,5-trisphosphate abbreviated InsP3 or Ins3P or IP3 is an inositol phosphate signaling molecule. It is made by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that is located in the plasma membrane, by phospholipase C (PLC). Together with diacylglycerol (DAG), IP3 is a second messenger molecule used in signal transduction in biological cells. While DAG stays inside the membrane, IP3 is soluble and diffuses through the cell, where it binds to its receptor, which is a calcium channel located in the endoplasmic reticulum. When IP3 binds its receptor, calcium is released into the cytosol, thereby activating various calcium regulated intracellular signals.

Sir Hans Adolf Krebs, FRS was a German-British biologist, physician and biochemist. He was a pioneer scientist in the study of cellular respiration, a biochemical process in living cells that extracts energy from food and oxygen and makes it available to drive the processes of life. He is best known for his discoveries of two important sequences of chemical reactions that take place in the cells of nearly all organisms, including humans, other than anaerobic microorganisms, namely the citric acid cycle and the urea cycle. The former, often eponymously known as the "Krebs cycle", is the sequence of metabolic reactions that allows cells of oxygen-respiring organisms to obtain far more ATP from the food they consume than anaerobic processes such as glycolysis can supply; and its discovery earned Krebs a Nobel Prize in Physiology or Medicine in 1953. With Hans Kornberg, he also discovered the glyoxylate cycle, a slight variation of the citric acid cycle found in plants, bacteria, protists, and fungi.
Wortmannin, a steroid metabolite of the fungi Penicillium funiculosum, Talaromyces wortmannii, is a non-specific, covalent inhibitor of phosphoinositide 3-kinases (PI3Ks). It has an in vitro inhibitory concentration (IC50) of around 5 nM, making it a more potent inhibitor than LY294002, another commonly used PI3K inhibitor. It displays a similar potency in vitro for the class I, II, and III PI3K members although it can also inhibit other PI3K-related enzymes such as mTOR, DNA-PKcs, some phosphatidylinositol 4-kinases, myosin light chain kinase (MLCK) and mitogen-activated protein kinase (MAPK) at high concentrations Wortmannin has also been reported to inhibit members of the polo-like kinase family with IC50 in the same range as for PI3K. The half-life of wortmannin in tissue culture is about 10 minutes due to the presence of the highly reactive C20 carbon that is also responsible for its ability to covalently inactivate PI3K. Wortmannin is a commonly used cell biology reagent that has been used previously in research to inhibit DNA repair, receptor-mediated endocytosis and cell proliferation.
In biochemistry, dephosphorylation is the removal of a phosphate (PO43−) group from an organic compound by hydrolysis. It is a reversible post-translational modification. Dephosphorylation and its counterpart, phosphorylation, activate and deactivate enzymes by detaching or attaching phosphoric esters and anhydrides. A notable occurrence of dephosphorylation is the conversion of ATP to ADP and inorganic phosphate.
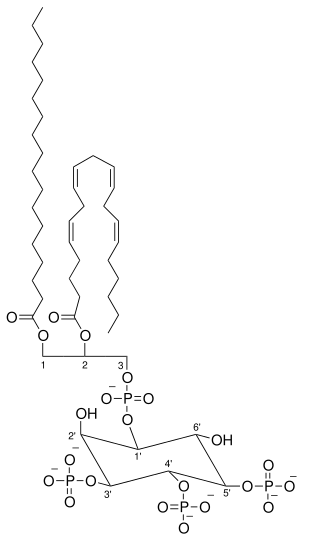
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases' (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.

Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer.
Sir Michael John Berridge (22 October 1938 - 13 February 2020) was a British physiologist and biochemist. He was known for his work on cell signaling, in particular the discovery that inositol trisphosphate acts as a second messenger, linking events at the plasma membrane with the release of calcium ions (Ca2+) within the cell.

Marie Maynard Daly was an American biochemist. She was the first African-American to receive a Ph.D. from Columbia University and the first African-American woman in the United States to earn a Ph.D. in chemistry. Daly made important contributions in four areas of research: the chemistry of histones, protein synthesis, the relationships between cholesterol and hypertension, and creatine's uptake by muscle cells.

Phosphatidylinositol 3-kinase regulatory subunit beta is an enzyme that in humans is encoded by the PIK3R2 gene.

Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role in eukaryotic cell physiology, in particular signal transduction pathways. Phospholipase C's role in signal transduction is its cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. Activators of each PLC vary, but typically include heterotrimeric G protein subunits, protein tyrosine kinases, small G proteins, Ca2+, and phospholipids.

Pyruvate dehydrogenase kinase isoform 2 (PDK2) also known as pyruvate dehydrogenase lipoamide kinase isozyme 2, mitochondrial is an enzyme that in humans is encoded by the PDK2 gene. PDK2 is an isozyme of pyruvate dehydrogenase kinase.

Phosphatidylinositol 4-kinase beta is an enzyme that in humans is encoded by the PI4KB gene.

PIKfyve, a FYVE finger-containing phosphoinositide kinase, is an enzyme that in humans is encoded by the PIKFYVE gene.

1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase delta-3 is an enzyme that in humans is encoded by the PLCD3 gene.

Phosphatidylinositol 4-kinase 2-alpha is an enzyme that in humans is encoded by the PI4K2A gene.

Chloride intracellular channel protein 5 is a protein that in humans is encoded by the CLIC5 gene.
Lewis C. Cantley is an American cell biologist and biochemist who has made significant advances to the understanding of cancer metabolism. Among his most notable contributions are the discovery and study of the enzyme PI-3-kinase, now known to be important to understanding cancer and diabetes mellitus. He is currently Meyer Director and Professor of Cancer Biology at the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine in New York City. He was formerly a professor in the Departments of Systems Biology and Medicine at Harvard Medical School, and the Director of Cancer Research at the Beth Israel Deaconess Medical Center, in Boston, Massachusetts. In 2016, he was elected Chairman of the Board for the Hope Funds for Cancer Research.
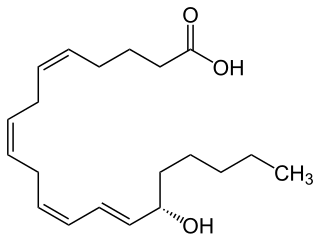
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5(S),15(S)-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE), a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.

Ronald Whittam was an English research scientist in the field of cell physiology. He conducted important studies on the mechanism of active transport of ions across animal membranes and its relation to cellular metabolism. Whittam was the inaugural Chair in Physiology at the University of Leicester, where he was subsequently an Emeritus Professor.
















