Related Research Articles
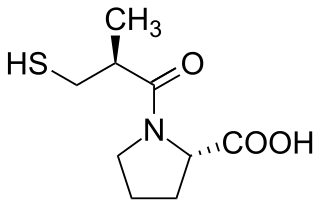
Angiotensin-converting-enzyme inhibitors are a class of medication used primarily for the treatment of high blood pressure and heart failure. This class of medicine works by causing relaxation of blood vessels as well as a decrease in blood volume, which leads to lower blood pressure and decreased oxygen demand from the heart.

Coronary artery disease (CAD), also called coronary heart disease (CHD), ischemic heart disease (IHD), myocardial ischemia, or simply heart disease, involves the reduction of blood flow to the heart muscle due to build-up of atherosclerotic plaque in the arteries of the heart. It is the most common of the cardiovascular diseases. Types include stable angina, unstable angina, and myocardial infarction.

Beta blockers, also spelled β-blockers, are a class of medications that are predominantly used to manage abnormal heart rhythms (arrhythmia), and to protect the heart from a second heart attack after a first heart attack. They are also widely used to treat high blood pressure, although they are no longer the first choice for initial treatment of most patients.
Drugs used in diabetes treat diabetes mellitus by decreasing the glucose level in the blood. With the exception of insulin, most GLP receptor agonists, and pramlintide, all are administered orally and are thus also called oral hypoglycemic agents or oral antihyperglycemic agents. There are different classes of hypoglycemic drugs, and their selection depends on the nature of diabetes, age, and situation of the person, as well as other factors.

The thiazolidinediones, abbreviated as TZD, also known as glitazones after the prototypical drug ciglitazone, are a class of heterocyclic compounds consisting of a five-membered C3NS ring. The term usually refers to a family of drugs used in the treatment of diabetes mellitus type 2 that were introduced in the late 1990s.

Rosiglitazone is an antidiabetic drug in the thiazolidinedione class. It works as an insulin sensitizer, by binding to the PPAR in fat cells and making the cells more responsive to insulin. It is marketed by the pharmaceutical company GlaxoSmithKline (GSK) as a stand-alone drug or for use in combination with metformin or with glimepiride. First released in 1999, annual sales peaked at approximately $2.5-billion in 2006; however, following a meta-analysis in 2007 that linked the drug's use to an increased risk of heart attack, sales plummeted to just $9.5-million in 2012. The drug's patent expired in 2012.

Pioglitazone, sold under the brand name Actos among others, is an anti-diabetic medication used to treat type 2 diabetes. It may be used with metformin, a sulfonylurea, or insulin. Use is recommended together with exercise and diet. It is not recommended in type 1 diabetes. It is taken by mouth.

Cardiac markers are biomarkers measured to evaluate heart function. They can be useful in the early prediction or diagnosis of disease. Although they are often discussed in the context of myocardial infarction, other conditions can lead to an elevation in cardiac marker level.

Acute coronary syndrome (ACS) is a syndrome due to decreased blood flow in the coronary arteries such that part of the heart muscle is unable to function properly or dies. The most common symptom is centrally located pressure-like chest pain, often radiating to the left shoulder or angle of the jaw, and associated with nausea and sweating. Many people with acute coronary syndromes present with symptoms other than chest pain, particularly women, older people, and people with diabetes mellitus.

P2Y12 is a chemoreceptor for adenosine diphosphate (ADP) that belongs to the Gi class of a group of G protein-coupled (GPCR) purinergic receptors. This P2Y receptor family has several receptor subtypes with different pharmacological selectivity, which overlaps in some cases, for various adenosine and uridine nucleotides. The P2Y12 receptor is involved in platelet aggregation and is thus a biological target for the treatment of thromboembolisms and other clotting disorders. Two transcript variants encoding the same isoform have been identified for this gene.

Bivalirudin (Bivalitroban), sold under the brand names Angiomax and Angiox and manufactured by The Medicines Company, is a specific and reversible direct thrombin inhibitor (DTI).
Troponin I is a cardiac and skeletal muscle protein family. It is a part of the troponin protein complex, where it binds to actin in thin myofilaments to hold the actin-tropomyosin complex in place. Troponin I prevents myosin from binding to actin in relaxed muscle. When calcium binds to the troponin C, it causes conformational changes which lead to dislocation of troponin I. Afterwards, tropomyosin leaves the binding site for myosin on actin leading to contraction of muscle. The letter I is given due to its inhibitory character. It is a useful marker in the laboratory diagnosis of heart attack. It occurs in different plasma concentration but the same circumstances as troponin T - either test can be performed for confirmation of cardiac muscle damage and laboratories usually offer one test or the other.
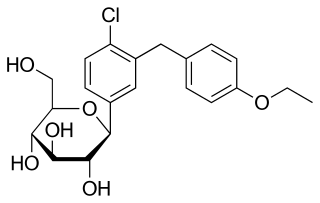
Dapagliflozin, sold under the brand names Farxiga (US) and Forxiga (EU) among others, is a medication used to treat type 2 diabetes. It is also used to treat adults with heart failure and chronic kidney disease.
Cardiac rehabilitation (CR) is defined by the World Health Organization (WHO) as "the sum of activity and interventions required to ensure the best possible physical, mental, and social conditions so that patients with chronic or post-acute cardiovascular disease may, by their own efforts, preserve or resume their proper place in society and lead an active life". CR is a comprehensive model of care delivering established core components, including structured exercise, patient education, psychosocial counselling, risk factor reduction and behaviour modification, with a goal of optimizing patient's quality of life and reducing the risk of future heart problems.

A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops in one of the coronary arteries of the heart, causing infarction to the heart muscle. The most common symptom is chest pain or discomfort which may travel into the shoulder, arm, back, neck or jaw. Often such pain occurs in the center or left side of the chest and lasts for more than a few minutes. The discomfort may occasionally feel like heartburn. Other symptoms may include shortness of breath, nausea, feeling faint, a cold sweat, feeling tired, and decreased level of consciousness. About 30% of people have atypical symptoms. Women more often present without chest pain and instead have neck pain, arm pain or feel tired. Among those over 75 years old, about 5% have had an MI with little or no history of symptoms. An MI may cause heart failure, an irregular heartbeat, cardiogenic shock or cardiac arrest.
Glucagon-like peptide-1 (GLP-1) receptor agonists, also known as GLP-1 analogs, GLP-1DAs or incretin mimetics, are a class of drugs that reduce blood sugar and energy intake by activating the GLP-1 receptor. They mimic the actions of the endogenous incretin hormone GLP-1 that is released by the gut after eating.

Canagliflozin, sold under the brand name Invokana among others, is a medication used to treat type 2 diabetes. It is used together with exercise and diet. It is not recommended in type 1 diabetes. It is taken by mouth.

Dulaglutide, sold under the brand name Trulicity among others, is a medication used for the treatment of type 2 diabetes in combination with diet and exercise. It is also approved in the United States for the reduction of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease or multiple cardiovascular risk factors. It is a once-weekly injection.
SGLT2 inhibitors, also called gliflozins or flozins, are a class of medications that inhibit sodium-glucose transport proteins in the nephron, unlike SGLT1 inhibitors that perform a similar function in the intestinal mucosa. The foremost metabolic effect of this is to inhibit reabsorption of glucose in the kidney and therefore lower blood sugar. They act by inhibiting sodium-glucose transport protein 2 (SGLT2). SGLT2 inhibitors are used in the treatment of type 2 diabetes. Apart from blood sugar control, gliflozins have been shown to provide significant cardiovascular benefit in people with type 2 diabetes. As of 2014, several medications of this class had been approved or were under development. In studies on canagliflozin, a member of this class, the medication was found to enhance blood sugar control as well as reduce body weight and systolic and diastolic blood pressure.
The volume of the heart's left atrium is an important biomarker for cardiovascular physiology and clinical cardiology. It is usually calculated as left atrial volume index in terms of body surface area.
References
- ↑ Bonora BM, Avogaro A, Fadini GP (2020). "Extraglycemic Effects of SGLT2 Inhibitors: A Review of the Evidence". Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy . 13: 161–174. doi: 10.2147/DMSO.S233538 . PMC 6982447 . PMID 32021362.
- ↑ Chong WH, Yanoff LB, Andraca-Carrera E, Hai MT (2020). "Assessing the Safety of Glucose-Lowering Drugs - A New Focus for the FDA". The New England Journal of Medicine . 383 (13): 1199–1202. doi:10.1056/NEJMp2004889. PMID 32966719. S2CID 221888300.
- ↑ Kip KE, Hollabaugh K, Marroquin OC, Williams DO (2008). "The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention". Journal of the American College of Cardiology . 51 (7): 701–707. doi: 10.1016/j.jacc.2007.10.034 . PMID 18279733.
- ↑ de Jong M, van der Worp HB, van der Graaf Y, Visseren FL, Westerink J (2017). "Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials". Cardiovascular Diabetology . 16 (1): 134. doi: 10.1186/s12933-017-0617-4 . PMC 5644073 . PMID 29037211.
- ↑ Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CS, Rodgers A, Mahaffey KW, Cannon CP, Perkovic V, Jardine MJ, Neal B (2020). "Sodium-Glucose Cotransporter 2 Inhibition for the Prevention of Cardiovascular Events in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis". Journal of the American Heart Association . 9 (3): e014908. doi:10.1161/JAHA.119.014908. PMC 7033896 . PMID 31992158.
- ↑ Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L (2017). "Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies". Journal of the American Heart Association . 6 (7): e004947. doi:10.1161/JAHA.116.004947. PMC 5586261 . PMID 28663251.
- ↑ Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM (2018). "Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease". PLOS One . 13 (6): e0198144. Bibcode:2018PLoSO..1398144R. doi: 10.1371/journal.pone.0198144 . PMC 5999069 . PMID 29897923.
- ↑ Poudel, I; Tejpal, C; Rashid, H; Jahan, N (30 July 2019). "Major Adverse Cardiovascular Events: An Inevitable Outcome of ST-elevation myocardial infarction? A Literature Review". Cureus. 11 (7): e5280. doi: 10.7759/cureus.5280 . PMC 6695291 . PMID 31423405. S2CID 201040946.
- ↑ Bonsu, JM; Guha, A; Charles, L; Yildiz, VO; Wei, L; Baker, B; Brammer, JE; Awan, F; Lustberg, M; Reinbolt, R; Miller, ED; Jneid, H; Ruz, P; Carter, RR; Milks, MW; Paskett, ED; Addison, D (18 February 2020). "Reporting of Cardiovascular Events in Clinical Trials Supporting FDA Approval of Contemporary Cancer Therapies". Journal of the American College of Cardiology. 75 (6): 620–628. doi:10.1016/j.jacc.2019.11.059. PMC 7860639 . PMID 32057377.
- ↑ Bosco, E; Hsueh, L; McConeghy, KW; Gravenstein, S; Saade, E (6 November 2021). "Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review". BMC Medical Research Methodology. 21 (1): 241. doi: 10.1186/s12874-021-01440-5 . PMC 8571870 . PMID 34742250. S2CID 243767377.
- ↑ Robertson, T; Beveridge, G; Bromley, C (2017). "Allostatic load as a predictor of all-cause and cause-specific mortality in the general population: Evidence from the Scottish Health Survey". PLOS ONE. 12 (8): e0183297. Bibcode:2017PLoSO..1283297R. doi: 10.1371/journal.pone.0183297 . PMC 5559080 . PMID 28813505.
- ↑ Zhao, X; Liu, C; Zhou, P; Sheng, Z; Li, J; Zhou, J; Chen, R; Wang, Y; Chen, Y; Song, L; Zhao, H; Yan, H (2020). "Estimation of Major Adverse Cardiovascular Events in Patients With Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A Risk Prediction Score Model From a Derivation and Validation Study". Frontiers in Cardiovascular Medicine. 7: 603621. doi: 10.3389/fcvm.2020.603621 . PMC 7728669 . PMID 33330667.
- ↑ Neumann, Johannes T.; Thao, Le T. P.; Callander, Emily; Chowdhury, Enayet; Williamson, Jeff D.; Nelson, Mark R.; Donnan, Geoffrey; Woods, Robyn L.; Reid, Christopher M.; Poppe, Katrina K.; Jackson, Rod; Tonkin, Andrew M.; McNeil, John J. (February 2022). "Cardiovascular risk prediction in healthy older people". GeroScience. 44 (1): 403–413. doi:10.1007/s11357-021-00486-z. PMC 8810999 . PMID 34762275.
- ↑ Chaker, L; van den Berg, ME; Niemeijer, MN; Franco, OH; Dehghan, A; Hofman, A; Rijnbeek, PR; Deckers, JW; Eijgelsheim, M; Stricker, BH; Peeters, RP (6 September 2016). "Thyroid Function and Sudden Cardiac Death: A Prospective Population-Based Cohort Study". Circulation. 134 (10): 713–22. doi: 10.1161/CIRCULATIONAHA.115.020789 . PMID 27601558. S2CID 207711411.
- ↑ Chaker, L; Baumgartner, C; den Elzen, WP; Collet, TH; Ikram, MA; Blum, MR; Dehghan, A; Drechsler, C; Luben, RN; Portegies, ML; Iervasi, G; Medici, M; Stott, DJ; Dullaart, RP; Ford, I; Bremner, A; Newman, AB; Wanner, C; Sgarbi, JA; Dörr, M; Longstreth WT, Jr; Psaty, BM; Ferrucci, L; Maciel, RM; Westendorp, RG; Jukema, JW; Ceresini, G; Imaizumi, M; Hofman, A; Bakker, SJ; Franklyn, JA; Khaw, KT; Bauer, DC; Walsh, JP; Razvi, S; Gussekloo, J; Völzke, H; Franco, OH; Cappola, AR; Rodondi, N; Peeters, RP; Thyroid Studies, Collaboration (November 2016). "Thyroid Function Within the Reference Range and the Risk of Stroke: An Individual Participant Data Analysis". The Journal of Clinical Endocrinology and Metabolism. 101 (11): 4270–4282. doi:10.1210/jc.2016-2255. PMC 5095234 . PMID 27603906.
- ↑ Müller, P; Dietrich, JW; Lin, T; Bejinariu, A; Binnebößel, S; Bergen, F; Schmidt, J; Müller, SK; Chatzitomaris, A; Kurt, M; Gerguri, S; Clasen, L; Klein, HH; Kelm, M; Makimoto, H (15 April 2020). "Usefulness of Serum Free Thyroxine Concentration to Predict Ventricular Arrhythmia Risk in Euthyroid Patients With Structural Heart Disease". The American Journal of Cardiology. 125 (8): 1162–1169. doi:10.1016/j.amjcard.2020.01.019. PMID 32087999. S2CID 211261823.
- ↑ Müller, P; Leow, MK; Dietrich, JW (2022). "Minor perturbations of thyroid homeostasis and major cardiovascular endpoints-Physiological mechanisms and clinical evidence". Frontiers in Cardiovascular Medicine. 9: 942971. doi: 10.3389/fcvm.2022.942971 . PMC 9420854 . PMID 36046184.
- 1 2 Zelniker TA, Wiviott SD, abatine MS (2019). "SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials". The Lancet . 393 (10166): 31–39. doi:10.1016/S0140-6736(18)32590-X. PMID 30424892. S2CID 53277899.
- ↑ Xu D, Chandler O, Xiao H (2021). "Sodium-Glucose Cotransporter-2 Inhibitor (SGLT2i) as a Primary Preventative Agent in the Healthy Individual: A Need of a Future Randomised Clinical Trial?". Frontiers in Medicine . 8: 712671. doi: 10.3389/fmed.2021.712671 . PMC 8419219 . PMID 34497814.
- ↑ Tippetts TS, Holland WL, Summers SA (2021). "Cholesterol - the devil you know; ceramide - the devil you don't". Trends in Pharmacological Sciences . 42 (12): 1082–1095. doi:10.1016/j.tips.2021.10.001. PMC 8595778 . PMID 34750017.