
Vancomycin is a glycopeptide antibiotic medication used to treat a number of bacterial infections. It is recommended intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant Staphylococcus aureus. Blood levels may be measured to determine the correct dose. Vancomycin is also taken by mouth as a treatment for severe Clostridium difficile colitis. When taken by mouth it is poorly absorbed.

Teicoplanin is an antibiotic used in the prophylaxis and treatment of serious infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Enterococcus faecalis. It is a semisynthetic glycopeptide antibiotic with a spectrum of activity similar to vancomycin. Its mechanism of action is to inhibit bacterial cell wall synthesis.

Vancomycin-resistant Staphylococcus aureus (VRSA) are strains of Staphylococcus aureus that have become resistant to the glycopeptide antibiotic vancomycin.

Glycopeptide antibiotics are a class of drugs of microbial origin that are composed of glycosylated cyclic or polycyclic nonribosomal peptides. Significant glycopeptide antibiotics include the anti-infective antibiotics vancomycin, teicoplanin, telavancin, ramoplanin and decaplanin, corbomycin, complestatin and the antitumor antibiotic bleomycin. Vancomycin is used if infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.

(S)-3,5-Dihydroxyphenylglycine or DHPG is a potent agonist of group I metabotropic glutamate receptors (mGluRs) mGluR1 and mGluR5.

Dalbavancin, sold under the brand names Dalvance in the US and Xydalba in the EU among others, is a second-generation lipoglycopeptide antibiotic medication. It belongs to the same class as vancomycin, the most widely used and one of the treatments available to people infected with methicillin-resistant Staphylococcus aureus (MRSA).

Efaroxan is an α2-adrenergic receptor antagonist and antagonist of the imidazoline receptor.

L-371,257 is a compound used in scientific research which acts as a selective antagonist of the oxytocin receptor with over 800x selectivity over the related vasopressin receptors. It was one of the first non-peptide oxytocin antagonists developed, and has good oral bioavailability, but poor penetration of the blood–brain barrier, which gives it good peripheral selectivity with few central side effects. Potential applications are likely to be in the treatment of premature labour.
Glycopeptides are peptides that contain carbohydrate moieties (glycans) covalently attached to the side chains of the amino acid residues that constitute the peptide.

Lipoglycopeptides are a class of antibiotic that have lipophilic side-chains linked to glycopeptides. The class includes oritavancin, telavancin and dalbavancin.

Biphalin is a dimeric enkephalin endogenous peptide (Tyr-D-Ala-Gly-Phe-NH)2 composed of two tetrapeptides derived from enkephalins, connected 'tail-to-tail' by a hydrazide bridge. The presence of two distinct pharmacophores confers on biphalin a high affinity for both μ and δ opioid receptors (with an EC50 of about 1-5 nM for both μ and δ receptors), therefore it has analgesic activity. Biphalin presents a considerable antinociceptive profile. In fact, when administered intracerebroventricularly in mice, biphalin displays a potency almost 7-fold greater than that of the ultra-potent alkaloid agonist, etorphine and 7000-fold greater than morphine; biphalin and morphine were found to be equipotent after intraperitoneal administration. The extraordinary in vivo potency shown by this compound is coupled with low side-effects, in particular, to produce no dependency in chronic use. For these reasons, several efforts have been carried out in order to obtain more information about structure-activity relationship (SAR). Results clearly indicate that, at least for μ receptor binding, the presence of two pharmacophores is not necessary; Tyr1 is indispensable for analgesic activity, while replacing Phe at the position 4 and 4' with non-aromatic, but lipophilic amino acids does not greatly change the binding properties and in general 4,4' positions are found to be important to design biphalin analogues with increased potency and modified μ/δ selectivity. The hydrazide linker is not fundamental for activity or binding, and it can be conveniently substituted by different conformationally constrained cycloaliphatic diamine linkers.
In molecular biology, VanY are protein domains found in enzymes named metallopeptidases. They are vital to bacterial cell wall synthesis and antibiotic resistance.
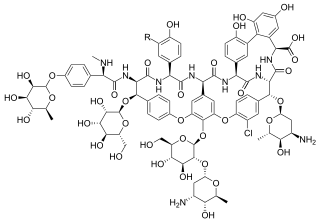
Avoparcin is a glycopeptide antibiotic effective against Gram-positive bacteria. It has been used in agriculture as an additive to livestock feed to promote growth in chickens, pigs, and cattle. It is also used as an aid in the prevention of necrotic enteritis in poultry.

CGS-13767 is an anxiolytic GABA receptor ligand.
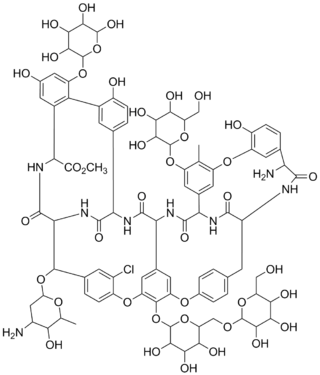
Actaplanin is a complex of broad-spectrum antibiotics made by Actinoplanes bacteria. Research carried out by a group in Eli Lilly and Co. in 1984 identified several actaplanins using high-performance liquid chromatography. Actaplanins A, B1, B2, B3, C1 and G were shown to be composed of the same peptide core, an amino sugar, and varying amounts of glucose, mannose, and rhamnose.
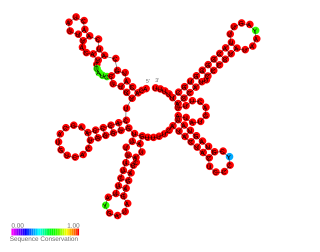
In molecular biology the small pathogenicity island RNA X gene is a bacterial non-coding RNA. It was discovered in a large-scale analysis of Staphylococcus aureus. SprX was shown to influence antibiotic resistance of the bacteria to Vancomycin and Teicoplanin glycopeptides, which are used to treat MRSA infections. In this study the authors identified a SprX target, stage V sporulation protein G. By reducing Spo VG expression levels, SprX affects S. aureus resistance to the glycopeptide antibiotics. Further work demonstrated its involvement in the regulation of pathogenicity factors.

4-Hydroxyphenylglycine (HPG) is a non-proteogenic amino acid found in vancomycin and related glycopeptides. HPG is synthesized from the shikimic acid pathway and requires four enzymes to synthesize: Both L- and D-HPG are used in the vancomycin class of antibiotics. Tyrosine, a similar amino acid, differs by a methylene group (CH2) between the aromatic ring and the alpha carbon.
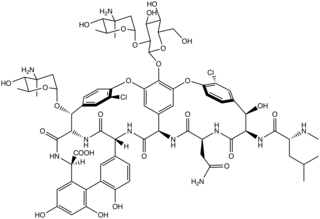
Chloroeremomycin is a member of the glycopeptide family of antibiotics, such as vancomycin. The molecule is a non-ribosomal polypeptide that has been glycosylated. It is composed of seven amino acids and three saccharide units. Although chloroeremomycin has never been in clinical phases, oritavancin, a semi-synthetic derivative of chloroeremomycin, has been investigated.
Corbomycin is a member of the glycopeptide family of antibiotics that are produced by soil bacteria.
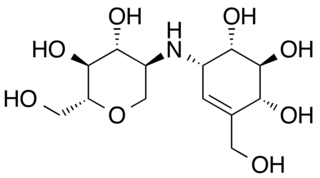
Salbostatin is an antibiotic and trehalase inhibitor with the molecular formula C13H23O8. Salbostatin is produced by the bacterium Streptomyces albus.
















