Related Research Articles

The endometrium is the inner epithelial layer, along with its mucous membrane, of the mammalian uterus. It has a basal layer and a functional layer: the basal layer contains stem cells which regenerate the functional layer. The functional layer thickens and then is shed during menstruation in humans and some other mammals, including other apes, Old World monkeys, some species of bat, the elephant shrew and the Cairo spiny mouse. In most other mammals, the endometrium is reabsorbed in the estrous cycle. During pregnancy, the glands and blood vessels in the endometrium further increase in size and number. Vascular spaces fuse and become interconnected, forming the placenta, which supplies oxygen and nutrition to the embryo and fetus. The speculated presence of an endometrial microbiota has been argued against.
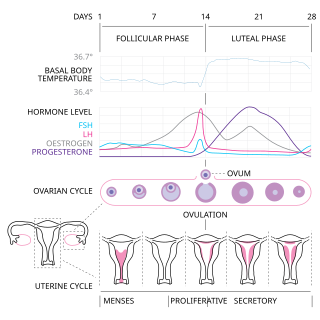
The menstrual cycle is a series of natural changes in hormone production and the structures of the uterus and ovaries of the female reproductive system that makes pregnancy possible. The ovarian cycle controls the production and release of eggs and the cyclic release of estrogen and progesterone. The uterine cycle governs the preparation and maintenance of the lining of the uterus (womb) to receive an embryo. These cycles are concurrent and coordinated, normally last between 21 and 35 days, with a median length of 28 days. Menarche usually occurs around the age of 12 years; menstrual cycles continue for about 30–45 years.

An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface.
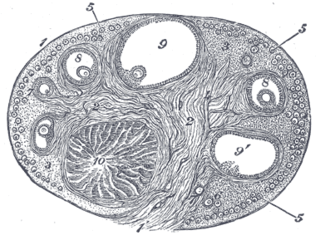
The corpus luteum is a temporary endocrine structure in female ovaries involved in the production of relatively high levels of progesterone, and moderate levels of estradiol, and inhibin A. It is the remains of the ovarian follicle that has released a mature ovum during a previous ovulation.
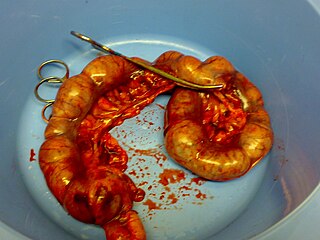
Pyometra or pyometritis is a uterine infection. Though it is most commonly known as a disease of the unaltered female dog, it is also a notable human disease. It is also seen in female cattle, horses, goats, sheep, swine, cats, rabbits, hamsters, ferrets, rats and guinea pigs. Pyometra is an important disease to be aware of for any dog or cat owner because of the sudden nature of the disease and the deadly consequences if left untreated. It has been compared to acute appendicitis in humans, because both are essentially empyemas within an abdominal organ.

Adenomyosis is a medical condition characterized by the growth of cells that proliferate on the inside of the uterus (endometrium) atypically located among the cells of the uterine wall (myometrium), as a result, thickening of the uterus occurs. As well as being misplaced in patients with this condition, endometrial tissue is completely functional. The tissue thickens, sheds and bleeds during every menstrual cycle.

Hysteroscopy is the inspection of the uterine cavity by endoscopy with access through the cervix. It allows for the diagnosis of intrauterine pathology and serves as a method for surgical intervention.

The human reproductive system includes the male reproductive system, which functions to produce and deposit sperm, and the female reproductive system, which functions to produce egg cells and to protect and nourish the fetus until birth. Humans have a high level of sexual differentiation. In addition to differences in nearly every reproductive organ, there are numerous differences in typical secondary sex characteristics.
The estrous cycle is a set of recurring physiological changes induced by reproductive hormones in females of mammalian subclass Theria. Estrous cycles start after sexual maturity in females and are interrupted by anestrous phases, otherwise known as "rest" phases, or by pregnancies. Typically, estrous cycles repeat until death. These cycles are widely variable in duration and frequency depending on the species. Some animals may display bloody vaginal discharge, often mistaken for menstruation. Many mammals used in commercial agriculture, such as cattle and sheep, may have their estrous cycles artificially controlled with hormonal medications for optimum productivity. The male equivalent, seen primarily in ruminants, is called rut.

Endometritis is inflammation of the inner lining of the uterus (endometrium). Symptoms may include fever, lower abdominal pain, and abnormal vaginal bleeding or discharge. It is the most common cause of infection after childbirth. It is also part of spectrum of diseases that make up pelvic inflammatory disease.
Bovine alphaherpesvirus 1 (BoHV-1) is a virus of the family Herpesviridae and the subfamily Alphaherpesvirinae, known to cause several diseases worldwide in cattle, including rhinotracheitis, vaginitis, balanoposthitis, abortion, conjunctivitis, and enteritis. BoHV-1 is also a contributing factor in shipping fever, also known as bovine respiratory disease (BRD). It is spread horizontally through sexual contact, artificial insemination, and aerosol transmission and it may also be transmitted vertically across the placenta. BoHV-1 can cause both clinical and subclinical infections, depending on the virulence of the strain. Although these symptoms are mainly non-life-threatening it is an economically important disease as infection may cause a drop in production and affect trade restrictions. Like other herpesviruses, BoHV-1 causes a lifelong latent infection and sporadic shedding of the virus. The sciatic nerve and trigeminal nerve are the sites of latency. A reactivated latent carrier is normally the source of infection in a herd. The clinical signs displayed are dependent on the virulence of the strain. There is a vaccine available which reduces the severity and incidence of disease. Some countries in Europe have successfully eradicated the disease by applying a strict culling policy.

Bovine viral diarrhea (BVD), bovine viral diarrhoea or mucosal disease, previously referred to as bovine virus diarrhea (BVD), is an economically significant disease of cattle that is found in the majority of countries throughout the world. Worldwide reviews of the economically assessed production losses and intervention programs incurred by BVD infection have been published. The causative agent, bovine viral diarrhea virus (BVDV), is a member of the genus Pestivirus of the family Flaviviridae.

Implantation, also known as nidation, is the stage in the mammalian embryonic development in which the blastocyst hatches, attaches, adheres, and invades into the endometrium of the female's uterus. Implantation is the first stage of gestation, and, when successful, the female is considered to be pregnant. An implanted embryo is detected by the presence of increased levels of human chorionic gonadotropin (hCG) in a pregnancy test. The implanted embryo will receive oxygen and nutrients in order to grow.
Streptolysins are two homogenous exotoxins from Streptococcus pyogenes. Types include streptolysin O, which is oxygen-labile, and streptolysin S, which is oxygen-stable.

Metritis is inflammation of the wall of the uterus, whereas endometritis is inflammation of the functional lining of the uterus, called the endometrium. The term pelvic inflammatory disease (PID) is often used for metritis.
Uterine serpins are members of the A clade of the serine protease inhibitor (serpin) superfamily of proteins and are encoded by the SERPINA14 gene. Uterine serpins are produced by the endometrium of a restricted group of mammals under the influence of progesterone or estrogen. These proteins appear to be inactive protease inhibitors and may function during pregnancy to regulate immune function or participate in transplacental transport.
Menstruation is the shedding of the uterine lining (endometrium). It occurs on a regular basis in uninseminated sexually reproductive-age females of certain mammal species.

The uterine microbiome refers to the community of commensal, nonpathogenic microorganisms—including bacteria, viruses, and yeasts/fungi—present in a healthy uterus, as well as in the amniotic fluid and endometrium. These microorganisms coexist in a specific environment within the uterus, playing a vital role in maintaining reproductive health. In the past, the uterus was believed to be a sterile environment, free of any microbial life. Recent advancements in microbiological research, particularly the improvement of 16S rRNA gene sequencing techniques, have challenged this long-held belief. These advanced techniques have made it possible to detect bacteria and other microorganisms present in very low numbers. Using this procedure that allows the detection of bacteria that cannot be cultured outside the body, studies of microbiota present in the uterus are expected to increase.

Bovine vaginal prolapse is a medical condition in cattle, characterised by an abnormally positioned (prolapsed) vagina. In most cases the bovine vaginal prolapse occurs near the time of calving, yet there are some examples of the vaginal prolapse in younger and non-pregnant animals. Another, but less common and more severe reproductive prolapse in cattle is so-called bovine uterine prolapse, where a uterus is the one being abnormally positioned.
References
- 1 2 "Professor Martin Sheldon". sites.google.com. Retrieved 31 May 2024.
- ↑ "Professor Martin Sheldon". www.swansea.ac.uk. Retrieved 27 May 2017.
- ↑ "2013 AMCB Distinquished[sic] Lecturer: Martin Sheldon" (PDF). Animal Molecular and Cellular Biology Graduate Program. Eleventh Annual Research Symposium. University of Florida.
- ↑ "Biography - Professor Martin Sheldon - Swansea University". sites.google.com. Retrieved 13 December 2019.
- ↑ "Annual Report 2005-06" (PDF). Royal Veterinary College, London.
- ↑ "Speakers' biographies". European Society for Reproductive Immunology. Retrieved 27 May 2017.
- ↑ "I Martin Sheldon FRCVS - Google Scholar Citations". scholar.google.co.uk. Retrieved 27 May 2017.
- ↑ American Journal of Reproductive Immunology - Editorial Board - Wiley Online Library
- ↑ Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ (December 2009). "Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle". Biology of Reproduction. 81 (6): 1025–32. doi:10.1095/biolreprod.109.077370. PMC 2784443 . PMID 19439727.
- ↑ Sheldon IM, Rycroft AN, Dogan B, Craven M, Bromfield JJ, Chandler A, et al. (February 2010). "Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice". PLOS ONE. 5 (2): e9192. Bibcode:2010PLoSO...5.9192S. doi: 10.1371/journal.pone.0009192 . PMC 2820550 . PMID 20169203.
- ↑ Amos MR, Healey GD, Goldstone RJ, Mahan SM, Düvel A, Schuberth HJ, Sandra O, Zieger P, Dieuzy-Labaye I, Smith DG, Sheldon IM (March 2014). "Differential endometrial cell sensitivity to a cholesterol-dependent cytolysin links Trueperella pyogenes to uterine disease in cattle". Biology of Reproduction. 90 (3): 54. doi: 10.1095/biolreprod.113.115972 . PMID 24478394.
- ↑ Ormsby TJ, Owens SE, Horlock AD, Davies D, Griffiths WJ, Wang Y, Cronin JG, Bromfield JJ, Sheldon IM (October 2021). "Oxysterols protect bovine endometrial cells against pore-forming toxins from pathogenic bacteria". FASEB Journal. 35 (10): e21889. doi: 10.1096/fj.202100036R . PMC 9272411 . PMID 34569656.
- ↑ WalesOnline (22 September 2010). "Welsh success in battle to stop causes of infertility". walesonline. Retrieved 27 May 2017.
- ↑ Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H (June 2002). "Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle". Reproduction. 123 (6): 837–45. doi: 10.1530/rep.0.1230837 . PMID 12052238.
- ↑ Price JC, Bromfield JJ, Sheldon IM (September 2013). "Pathogen-associated molecular patterns initiate inflammation and perturb the endocrine function of bovine granulosa cells from ovarian dominant follicles via TLR2 and TLR4 pathways". Endocrinology. 154 (9): 3377–86. doi: 10.1210/en.2013-1102 . PMID 23825132.
- ↑ Preta G, Lotti V, Cronin JG, Sheldon IM (April 2015). "Protective role of the dynamin inhibitor Dynasore against the cholesterol-dependent cytolysin of Trueperella pyogenes". FASEB Journal. 29 (4): 1516–28. doi: 10.1096/fj.14-265207 . PMC 4396600 . PMID 25550455.
- ↑ Griffin S, Healey GD, Sheldon IM (October 2018). "Isoprenoids increase bovine endometrial stromal cell tolerance to the cholesterol-dependent cytolysin from Trueperella pyogenes". Biology of Reproduction. 99 (4): 749–760. doi:10.1093/biolre/ioy099. PMC 6203874 . PMID 29688258.
- ↑ "Celebration and change at RCVS Day 2013". RCVS. Retrieved 27 May 2017.
- ↑ "Iamnews.co.uk".