
A membrane electrode assembly (MEA) is an assembled stack of proton-exchange membranes (PEM) or alkali anion exchange membrane (AAEM), catalyst and flat plate electrode used in fuel cells and electrolyzers. [1] [2]

A membrane electrode assembly (MEA) is an assembled stack of proton-exchange membranes (PEM) or alkali anion exchange membrane (AAEM), catalyst and flat plate electrode used in fuel cells and electrolyzers. [1] [2]

The PEM is sandwiched between two electrodes which have the catalyst embedded in them. The electrodes are electrically insulated from each other by the PEM. These two electrodes make up the anode and cathode respectively.
The PEM is typically a fluoropolymer (PFSA) proton permeable electrical insulator barrier. Hydrocarbon variants are currently being developed and are expected to succeed fluoropolymers. This barrier allows the transport of the protons from the anode to the cathode through the membrane but forces the electrons to travel around a conductive path to the cathode. The most commonly used Nafion PEMs are Nafion XL, 112, 115, 117, and 1110.
The electrodes are heat pressed onto the PEM. Commonly used materials for these electrodes are carbon cloth or carbon fiber papers. [3] NuVant produces a carbon cloth called ELAT which maximizes gas transport to the PEM as well as moves water vapor away from the PEM. Imbedding ELAT with noble metal catalyst allows this carbon cloth to also act as the electrode. Many other different methods and procedures also exist for the production of MEAs which are quite similar between fuel cells and electrolyzers. [1]
Platinum is one of the most commonly used catalysts, however other platinum group metals are also used. Ruthenium and platinum are often used together, if carbon monoxide (CO) is a product of the electro-chemical reaction as CO poisons the PEM and impacts the efficiency of the fuel cell. Due to the high cost of these and other similar materials, research is being undertaken to develop catalysts that use lower cost materials as the high costs are still a hindering factor in the widespread economical acceptance of fuel cell technology.
Current service life is 7,300 hours under cycling conditions, while at the same time reducing platinum group metal loading to 0.2 mg/cm2. [4]
At this time most companies manufacturing MEAs specialize solely in high volume production, such as W. L. Gore & Associates, Johnson Matthey, and 3M. However, there are other companies which produce MEAs, allowing different shapes, catalysts or membranes to be evaluated as well, which include De Nora Tech, Fuel Cell Store, FuelCellsEtc, and many others.
The global market for Membrane Electrode Assemblies (MEA) was estimated to be worth US$ 672 million in 2023 and is forecast to reach US$ 3853 million by 2030, with a CAGR of 28.2% during the forecast period 2024-2030. [5]

A fuel cell is an electrochemical cell that converts the chemical energy of a fuel and an oxidizing agent into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity."
A regenerative fuel cell or reverse fuel cell (RFC) is a fuel cell run in reverse mode, which consumes electricity and chemical B to produce chemical A. By definition, the process of any fuel cell could be reversed. However, a given device is usually optimized for operating in one mode and may not be built in such a way that it can be operated backwards. Standard fuel cells operated backwards generally do not make very efficient systems unless they are purpose-built to do so as with high-pressure electrolysers, regenerative fuel cells, solid-oxide electrolyser cells and unitized regenerative fuel cells.

Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer-copolymer synthesized in 1962 by Dr. Donald J. Connolly at the DuPont Experimental Station in Wilmington Delaware. Additional work on the polymer family was performed in the late 1960s by Dr. Walther Grot of DuPont. Nafion is a brand of the Chemours company. It is the first of a class of synthetic polymers with ionic properties that are called ionomers. Nafion's unique ionic properties are a result of incorporating perfluorovinyl ether groups terminated with sulfonate groups onto a tetrafluoroethylene (PTFE) backbone. Nafion has received a considerable amount of attention as a proton conductor for proton exchange membrane (PEM) fuel cells because of its excellent chemical and mechanical stability in the harsh conditions of this application.
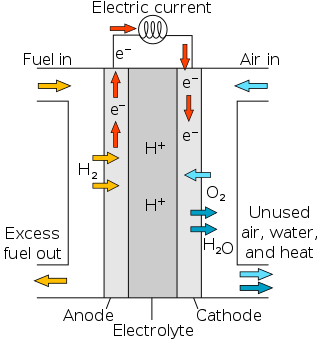
Proton-exchange membrane fuel cells (PEMFC), also known as polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging alkaline fuel-cell technology, which was used in the Space Shuttle.

Direct methanol fuel cells or DMFCs are a subcategory of proton-exchange membrane fuel cells in which methanol is used as the fuel and a special proton-conducting polymer as the membrane (PEM). Their main advantage is low temperature operation and the ease of transport of methanol, an energy-dense yet reasonably stable liquid at all environmental conditions.

The alkaline fuel cell (AFC), also known as the Bacon fuel cell after its British inventor, Francis Thomas Bacon, is one of the most developed fuel cell technologies. Alkaline fuel cells consume hydrogen and pure oxygen, to produce potable water, heat, and electricity. They are among the most efficient fuel cells, having the potential to reach 70%.
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen gas. This is their essential function when incorporated into a membrane electrode assembly (MEA) of a proton-exchange membrane fuel cell or of a proton-exchange membrane electrolyser: separation of reactants and transport of protons while blocking a direct electronic pathway through the membrane.

Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C.
Direct-ethanol fuel cells or DEFCs are a category of fuel cell in which ethanol is fed directly into the cell. They have been used as a model to investigate a range of fuel cell concepts including the use of PEM.
Platinum black is a fine powder of platinum with good catalytic properties. The name of platinum black is due to its black color. It is used in many ways; as a thin film electrode, a fuel cell membrane catalyst, or as a catalytic ignition of flammable gases for "self-lighting' gas lamps, ovens, and stove burners.

Reformed Methanol Fuel Cell (RMFC) or Indirect Methanol Fuel Cell (IMFC) systems are a subcategory of proton-exchange fuel cells where, the fuel, methanol (CH3OH), is reformed, before being fed into the fuel cell.
Gas diffusion electrodes (GDE) are electrodes with a conjunction of a solid, liquid and gaseous interface, and an electrical conducting catalyst supporting an electrochemical reaction between the liquid and the gaseous phase.
An electrochemical hydrogen compressor is a hydrogen compressor where hydrogen is supplied to the anode, and compressed hydrogen is collected at the cathode with an exergy efficiency up to and even beyond 80% for pressures up to 10,000 psi or 700 bars.
Membraneless Fuel Cells convert stored chemical energy into electrical energy without the use of a conducting membrane as with other types of Fuel Cells. In Laminar flow fuel cells (LFFC) this is achieved by exploiting the phenomenon of non-mixing laminar flows where the interface between the two flows works as a proton/ion conductor. The interface allows for high diffusivity and eliminates the need for costly membranes. The operating principles of these cells mean that they can only be built to millimeter-scale sizes. The lack of a membrane means they are cheaper but the size limits their use to portable applications which require small amounts of power.

An alkaline anion-exchange membrane fuel cell (AAEMFC), also known as anion-exchange membrane fuel cells (AEMFCs), alkaline membrane fuel cells (AMFCs), hydroxide-exchange membrane fuel cells (HEMFCs), or solid alkaline fuel cells (SAFCs) is a type of alkaline fuel cell that uses an anion-exchange membrane to separate the anode and cathode compartments.
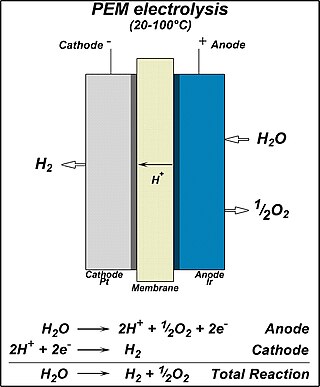
Proton exchange membrane(PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer. It involves a proton-exchange membrane.
Solid acid fuel cells (SAFCs) are a class of fuel cells characterized by the use of a solid acid material as the electrolyte. Similar to proton exchange membrane fuel cells and solid oxide fuel cells, they extract electricity from the electrochemical conversion of hydrogen- and oxygen-containing gases, leaving only water as a byproduct. Current SAFC systems use hydrogen gas obtained from a range of different fuels, such as industrial-grade propane and diesel. They operate at mid-range temperatures, from 200 to 300 °C.
Hydrogen evolution reaction (HER) is a chemical reaction that yields H2. The conversion of protons to H2 requires reducing equivalents and usually a catalyst. In nature, HER is catalyzed by hydrogenase enzymes. Commercial electrolyzers typically employ supported platinum as the catalyst at the anode of the electrolyzer. HER is useful for producing hydrogen gas, providing a clean-burning fuel. HER, however, can also be an unwelcome side reaction that competes with other reductions such as nitrogen fixation, or electrochemical reduction of carbon dioxide or chrome plating.

Anion exchange membrane(AEM) electrolysis is the electrolysis of water that utilises a semipermeable membrane that conducts hydroxide ions (OH−) called an anion exchange membrane. Like a proton-exchange membrane (PEM), the membrane separates the products, provides electrical insulation between electrodes, and conducts ions. Unlike PEM, AEM conducts hydroxide ions. The major advantage of AEM water electrolysis is that a high-cost noble metal catalyst is not required, low-cost transition metal catalyst can be used instead. AEM electrolysis is similar to alkaline water electrolysis, which uses a non-ion-selective separator instead of an anion-exchange membrane.