
Mesothelioma is a type of cancer that develops from the thin layer of tissue that covers many of the internal organs. The area most commonly affected is the lining of the lungs and chest wall. Less commonly the lining of the abdomen and rarely the sac surrounding the heart, or the sac surrounding the testis may be affected. Signs and symptoms of mesothelioma may include shortness of breath due to fluid around the lung, a swollen abdomen, chest wall pain, cough, feeling tired, and weight loss. These symptoms typically come on slowly.
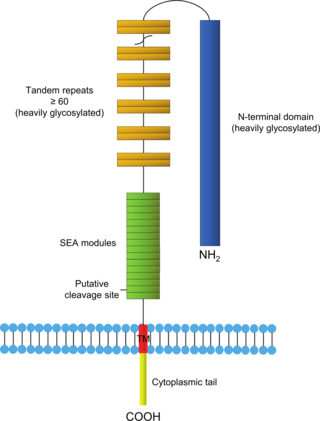
Mucin-16(MUC-16) also known as Ovarian cancer-related tumor marker CA125 is a protein that in humans is encoded by the MUC16 gene. MUC-16 is a member of the mucin family glycoproteins. MUC-16 has found application as a tumor marker or biomarker that may be elevated in the blood of some patients with specific types of cancers, most notably ovarian cancer, or other conditions that are benign.

Hybridoma technology is a method for producing large numbers of identical antibodies, also called monoclonal antibodies. This process starts by injecting a mouse with an antigen that provokes an immune response. A type of white blood cell, the B cell, produces antibodies that bind to the injected antigen. These antibody producing B-cells are then harvested from the mouse and, in turn, fused with immortal myeloma cancer cells, to produce a hybrid cell line called a hybridoma, which has both the antibody-producing ability of the B-cell and the longevity and reproductivity of the myeloma.

Carcinoembryonic antigen (CEA) describes a set of highly-related glycoproteins involved in cell adhesion. CEA is normally produced in gastrointestinal tissue during fetal development, but the production stops before birth. Consequently, CEA is usually present at very low levels in the blood of healthy adults. However, the serum levels are raised in some types of cancer, which means that it can be used as a tumor marker in clinical tests. Serum levels can also be elevated in heavy smokers.

Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective, to induce a specific cell signal, to cause the immune system to attack specific cells, or to bring a drug to a specific cell type.

Glypicans constitute one of the two major families of heparan sulfate proteoglycans, with the other major family being syndecans. Six glypicans have been identified in mammals, and are referred to as GPC1 through GPC6. In Drosophila two glypicans have been identified, and these are referred to as dally and dally-like. One glypican has been identified in C. elegans. Glypicans seem to play a vital role in developmental morphogenesis, and have been suggested as regulators for the Wnt and Hedgehog cell signaling pathways. They have additionally been suggested as regulators for fibroblast growth factor and bone morphogenic protein signaling.

Ira Pastan is an American scientist at the National Cancer Institute. He is a member of the National Academy of Sciences, a Fellow of the AAAS and the American Society of Microbiology. In 2009, he was awarded the prestigious International Antonio Feltrinelli Prize for Medicine. His wife, Linda Pastan, was an American poet.

CD47 also known as integrin associated protein (IAP) is a transmembrane protein that in humans is encoded by the CD47 gene. CD47 belongs to the immunoglobulin superfamily and partners with membrane integrins and also binds the ligands thrombospondin-1 (TSP-1) and signal-regulatory protein alpha (SIRPα). CD-47 acts as a don't eat me signal to macrophages of the immune system which has made it a potential therapeutic target in some cancers, and more recently, for the treatment of pulmonary fibrosis.

Glypican-3 is a protein that, in humans, is encoded by the GPC3 gene. The GPC3 gene is located on human X chromosome (Xq26) where the most common gene encodes a 70-kDa core protein with 580 amino acids. Three variants have been detected that encode alternatively spliced forms termed Isoforms 1 (NP_001158089), Isoform 3 (NP_001158090) and Isoform 4 (NP_001158091).

Glypican-1 (GPC1) is a protein that in humans is encoded by the GPC1 gene. GPC1 is encoded by human GPC1 gene located at 2q37.3. GPC1 contains 558 amino acids with three predicted heparan sulfate chains.

Cluster of Differentiation 276 (CD276) or B7 Homolog 3 (B7-H3) is a human protein encoded by the CD276 gene.
A431 cells are a model human cell line used in biomedical research.
CA 242 is a tumor marker for sialylated Lewis carbohydrates associated with adenocarcinomas and e-selectin-mediated metastatic risk. It is commonly tested along with CEA, CA19-9, and CA242 for detecting pancreatic cancer. The specificity of CA 242 is higher than similar markers. Current research dictates that diagnostic efficiency is highest when various tumor markers are tested for at once.
Folate targeting is a method utilized in biotechnology for drug delivery purposes. This Trojan Horse process, which was created by Drs. Christopher P. Leamon and Philip S. Low, involves the attachment of the vitamin, folate, to a molecule/drug to form a "folate conjugate". Based on the natural high affinity of folate for the folate receptor protein (FR), which is commonly expressed on the surface of many human cancers, folate-drug conjugates also bind tightly to the FR and trigger cellular uptake via endocytosis. Molecules as diverse as small radiodiagnostic imaging agents to large DNA plasmid formulations have successfully been delivered inside FR-positive cells and tissues.
A rabbit hybridoma is a hybrid cell line formed by the fusion of an antibody producing rabbit B cell with a cancerous B-cell (myeloma).
Amatuximab is a chimeric monoclonal antibody designed for the treatment of cancer. It was developed by Morphotek, Inc.
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.

A cancer biomarker refers to a substance or process that is indicative of the presence of cancer in the body. A biomarker may be a molecule secreted by a tumor or a specific response of the body to the presence of cancer. Genetic, epigenetic, proteomic, glycomic, and imaging biomarkers can be used for cancer diagnosis, prognosis, and epidemiology. Ideally, such biomarkers can be assayed in non-invasively collected biofluids like blood or serum.
GL-ONC1 is an investigational therapeutic product consisting of the clinical grade formulation of the laboratory strain GLV-1h68, an oncolytic virus developed by Genelux Corporation. GL-ONC1 is currently under evaluation in Phase I/II human clinical trials in the United States and Europe.
Thymidine kinase is an enzyme, a phosphotransferase : 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, EC 2.7.1.21 that catalyzes the reaction:
















