In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R-(C:)-R' or R=C: where the R represent substituents or hydrogen atoms.
The diazogroup is an organic moiety consisting of two linked nitrogen atoms (azo) at the terminal position. Overall charge neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula R2C=N+=N–. The simplest example of a diazo compound is diazomethane, CH2N2. Diazo compounds (R2C=N2) should not be confused with azo compounds of the type R-N=N-R or with diazonium compounds of the type R-N2+.
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).

The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.
An isocyanide is an organic compound with the functional group -N≡C. It is the isomer of the related nitrile (-C≡N), hence the prefix is isocyano. The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds.
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been reported. Many methods for synthesizing them and reactions utilizing them have been reported. The term carbene ligand is a formalism since many are not derived from carbenes and almost none exhibit the reactivity characteristic of carbenes. Described often as M=CR2, they represent a class of organic ligands intermediate between alkyls (−CR3) and carbynes (≡CR). They feature in some catalytic reactions, especially alkene metathesis, and are of value in the preparation of some fine chemicals.

Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It is a red solid that is pyrophoric in the air, and thus is typically handled with air-free techniques. It was originally synthesized by Fred Tebbe at DuPont Central Research.
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

Cyclopropanation refers to any chemical process which generates cyclopropane rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroids and a number of quinolone antibiotics. However the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions. Many of the reactions proceed in a cheletropic manner.

Organotitanium compounds in organometallic chemistry contain carbon-to-titanium chemical bonds. Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry and are involved in major industrial processes.
The Kulinkovich reaction describes the organic synthesis of cyclopropanols via reaction of esters with dialkyldialkoxytitanium reagents, generated in situ from Grignard reagents bearing hydrogen in beta-position and titanium(IV) alkoxides such as titanium isopropoxide. This reaction was first reported by Oleg Kulinkovich and coworkers in 1989.
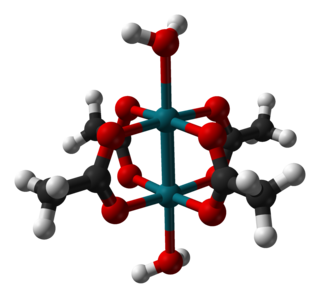
Rhodium(II) acetate is the coordination compound with the formula Rh2(AcO)4, where AcO− is the acetate ion (CH
3CO−
2). This dark green powder is slightly soluble in polar solvents, including water. It is used as a catalyst for cyclopropanation of alkenes. It is a widely studied example of a transition metal carboxylate complex.

Methyl phenylacetate is an organic compound that is the methyl ester of phenylacetic acid, with the structural formula C6H5CH2COOCH3. It is a colorless liquid that is only slightly soluble in water, but soluble in most organic solvents.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Iron adopts oxidation states from Fe(−II) through to Fe(VII). Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Carbene C−H insertion in organic chemistry concerns the insertion reaction of a carbene into a carbon–hydrogen bond. This organic reaction is of some importance in the synthesis of new organic compounds.
Metal-catalyzed intermolecular carbenoid cyclopropanations are organic reactions that result in the formation of a cyclopropane ring from a metal carbenoid specie and an alkene. In the Simmons–Smith reaction the metal involved is zinc.
The Buchner ring expansion is a two-step organic C-C bond forming reaction used to access 7-membered rings. The first step involves formation of a carbene from ethyl diazoacetate, which cyclopropanates an aromatic ring. The ring expansion occurs in the second step, with an electrocyclic reaction opening the cyclopropane ring to form the 7-membered ring.

Carbene radicals are a special class of organometallic carbenes. The carbene radical can be formed by one-electron reduction of Fischer-type carbenes using an external reducing agent, or directly upon carbene formation at an open-shell transition metal complex using diazo compounds and related carbene precursors. Cobalt(III)-carbene radicals have found catalytic applications in cyclopropanation reactions, as well as in a variety of other catalytic radical-type ring-closing reactions.
Cobalt(II)–porphyrin catalysis is a process in which a Co(II) porphyrin complex acts as a catalyst, inducing and accelerating a chemical reaction.
A Fischer carbene is an organometallic compound featuring a divalent organic ligand which is a strong π-acceptors at the metal and being electrophilic at the carbene carbon atom.









