
Apoptosis is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, each day the approximate lost is 20 to 30 billion cells.

Selenium is a chemical element with the symbol Se and atomic number 34. It is a metalloid with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

Vitamin B6 is one of the B vitamins, and thus an essential nutrient. The term refers to a group of six chemically similar compounds, i.e., "vitamers", which can be interconverted in biological systems. Its active form, pyridoxal 5′-phosphate, serves as a coenzyme in more than 140 enzyme reactions in amino acid, glucose, and lipid metabolism.

Astaxanthin is a keto-carotenoid within a group of chemical compounds known as terpenes. Astaxanthin is a metabolite of zeaxanthin and canthaxanthin, containing both hydroxyl and ketone functional groups. It is a lipid-soluble pigment with red coloring properties, which result from the extended chain of conjugated double bonds at the center of the compound.

Selenomethionine (SeMet) is a naturally occurring amino acid. The L-selenomethionine enantiomer is the main form of selenium found in Brazil nuts, cereal grains, soybeans, and grassland legumes, while Se-methylselenocysteine, or its γ-glutamyl derivative, is the major form of selenium found in Astragalus, Allium, and Brassica species. In vivo, selenomethionine is randomly incorporated instead of methionine. Selenomethionine is readily oxidized.

Sodium selenite is the inorganic compound with the formula Na2SeO3. This salt is a colourless solid. The pentahydrate Na2SeO3(H2O)5 is the most common water-soluble selenium compound.
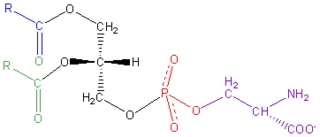
Phosphatidylserine is a phospholipid and is a component of the cell membrane. It plays a key role in cell cycle signaling, specifically in relation to apoptosis. It is a key pathway for viruses to enter cells via apoptotic mimicry. Its exposure on the outer surface of a membrane marks the cell for destruction via apoptosis.
Deguelin is a derivative of rotenone. Both are compounds classified as rotenoids of the flavonoid family and are naturally occurring insecticides. They can be produced by extraction from several plant species belonging to three genera of the legume family, Fabaceae: Lonchocarpus, Derris, or Tephrosia.

The BH3 interacting-domain death agonist, or BID, gene is a pro-apoptotic member of the Bcl-2 protein family. Bcl-2 family members share one or more of the four characteristic domains of homology entitled the Bcl-2 homology (BH) domains, and can form hetero- or homodimers. Bcl-2 proteins act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities.

Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5, is a protein that, in humans, is encoded by the BIRC5 gene.
Pyroptosis is a highly inflammatory form of lytic programmed cell death that occurs most frequently upon infection with intracellular pathogens and is likely to form part of the antimicrobial response. This process promotes the rapid clearance of various bacterial, viral, fungal and protozoan infections by removing intracellular replication niches and enhancing the host's defensive responses. Pyroptosis can take place in immune cells and is also reported to occur in keratinocytes and some epithelial cells.

Caspase-8 is a caspase protein, encoded by the CASP8 gene. It most likely acts upon caspase-3. CASP8 orthologs have been identified in numerous mammals for which complete genome data are available. These unique orthologs are also present in birds.

Serine/threonine-protein kinase 4 is an enzyme that in humans is encoded by the STK4 gene.

Diablo homolog (DIABLO) is a mitochondrial protein that in humans is encoded by the DIABLO gene on chromosome 12. DIABLO is also referred to as second mitochondria-derived activator of caspases or SMAC. This protein binds inhibitor of apoptosis proteins (IAPs), thus freeing caspases to activate apoptosis. Due to its proapoptotic function, SMAC is implicated in a broad spectrum of tumors, and small molecule SMAC mimetics have been developed to enhance current cancer treatments.
Selenium deficiency occurs when an organism lacks the required levels of selenium, a critical nutrient in many species. Deficiency, although relatively rare in healthy well-nourished individuals, can have significant negative results, affecting the health of the heart and the nervous system; contributing to depression, anxiety, and dementia; and interfering with reproduction and gestation.
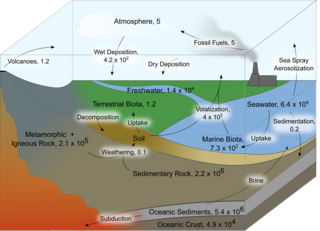
Iodine is an essential trace element in biological systems. It has the distinction of being the heaviest element commonly needed by living organisms as well as the second-heaviest known to be used by any form of life. It is a component of biochemical pathways in organisms from all biological kingdoms, suggesting its fundamental significance throughout the evolutionary history of life.
Selenium yeast is a feed additive for livestock, used to increase the selenium content in their fodder. It is a form of selenium currently approved for human consumption in the EU and Britain. Inorganic forms of selenium are used in feeds. Since these products can be patented, producers can demand premium prices. It is produced by fermenting Saccharomyces cerevisiae in a selenium-rich media.

Selenium is an essential micronutrient for animals, though it is toxic in large doses. In plants, it sometimes occurs in toxic amounts as forage, e.g. locoweed. Selenium is a component of the amino acids selenocysteine and selenomethionine. In humans, selenium is a trace element nutrient that functions as cofactor for glutathione peroxidases and certain forms of thioredoxin reductase. Selenium-containing proteins are produced from inorganic selenium via the intermediacy of selenophosphate (PSeO33−).

Methaneseleninic acid (methylseleninic acid or MSA) is a seleninic acid with the chemical formula CH3SeO2H.

Dicycloplatin is a chemotherapy medication used to treat a number of cancers which includes the non-small-cell lung carcinoma and prostate cancer.

















