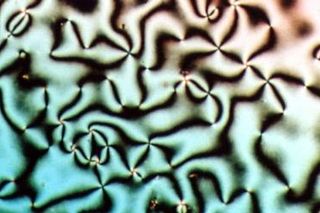
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties. The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word "surfactant" is a blend of surface-active agent, coined in 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt.

A micelle or micella is an aggregate of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension. A typical micelle in water forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre.

In chemistry, an amphiphile, or amphipath, is a chemical compound possessing both hydrophilic and lipophilic properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic compounds include surfactants and detergents. The phospholipid amphiphiles are the major structural component of cell membranes.

Dipalmitoylphosphatidylcholine (DPPC) is a phospholipid (and a lecithin) consisting of two C16 palmitic acid groups attached to a phosphatidylcholine head-group.
Poloxamers are nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene flanked by two hydrophilic chains of polyoxyethylene. The word poloxamer was coined by BASF inventor, Irving Schmolka, who received the patent for these materials in 1973. Poloxamers are also known by the trade names Pluronic, Kolliphor, and Synperonic.
Micellar liquid chromatography (MLC) is a form of reversed phase liquid chromatography that uses an aqueous micellar solutions as the mobile phase.

In biophysics and colloidal chemistry, polymorphism is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of spheres of lipid molecules (micelles), pairs of layers that face one another, a tubular arrangement (hexagonal), or various cubic phases. More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases.
In chemistry, bolaamphiphiles are amphiphilic molecules that have hydrophilic groups at both ends of a sufficiently long hydrophobic hydrocarbon chain. Compared to single-headed amphiphiles, the introduction of a second head-group generally induces a higher solubility in water, an increase in the critical micelle concentration (CMC), and a decrease in aggregation number. The aggregate morphologies of bolaamphiphiles include spheres, cylinders, disks, and vesicles. Bolaamphiphiles are also known to form helical structures that can form monolayer microtubular self-assemblies.
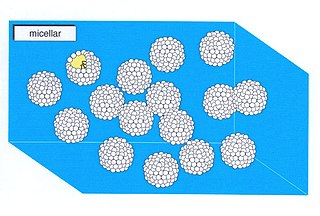
In colloid science, a micellar solution consists of a dispersion of micelles in a solvent. Micelles are made of chemicals that are attracted to both water and oily solvents, known as amphiphiles. In a micellar solution, some amphiphiles are clumped together and some are dispersed. Micellar solutions form when the concentration of amphiphile exceeds the critical micelle concentration (CMC) or critical aggregation concentration (CAC), which is when there are enough amphiphiles in the solution to clump together to form micells. Micellar solutions persist until the amphiphile concentration becomes sufficiently high to form a lyotropic liquid crystal phase.
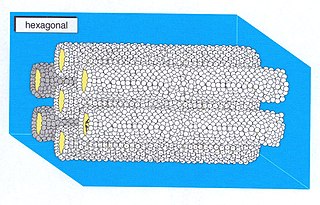
A hexagonal phase of lyotropic liquid crystal is formed by some amphiphilic molecules when they are mixed with water or another polar solvent. In this phase, the amphiphile molecules are aggregated into cylindrical structures of indefinite length and these cylindrical aggregates are disposed on a hexagonal lattice, giving the phase long-range orientational order.

Lyotropic liquid crystals result when amphiphiles, which are both hydrophobic and hydrophilic, dissolve into a solution that behaves both like a liquid and a solid crystal. This liquid crystalline mesophase includes everyday mixtures like soap and water.
Sodium lauroyl sarcosinate (INCI), also known as sarcosyl, is an anionic surfactant derived from sarcosine used as a foaming and cleansing agent in shampoo, shaving foam, toothpaste, and foam wash products.
A Ramsden emulsion, sometimes named Pickering emulsion, is an emulsion that is stabilized by solid particles which adsorb onto the interface between the water and oil phases. Typically, the emulsions are either water-in-oil or oil-in-water emulsions, but other more complex systems such as water-in-water, oil-in-oil, water-in-oil-in-water, and oil-in-water-in-oil also do exist. Pickering emulsions were named after S.U. Pickering, who described the phenomenon in 1907, although the effect was first recognized by Walter Ramsden in 1903.

Lauryldimethylamine oxide (LDAO), also known as dodecyldimethylamine oxide (DDAO), is an amine oxide–based zwitterionic surfactant, with a C12 (dodecyl) alkyl tail. It is one of the most frequently-used surfactants of this type. Like other amine oxide–based surfactants it is antimicrobial, being effective against common bacteria such as S. aureus and E. coli, however, it is also non-denaturing and may be used to solubilize proteins.
Surface rheology is a description of the rheological properties of a free surface. When perfectly pure, the interface between fluids usually displays only surface tension. The stress within a fluid interface can be affected by the adsorption of surfactants in several ways:
In colloidal chemistry, the critical micelle concentration (CMC) of a surfactant is one of the parameters in the Gibbs free energy of micellization. The concentration at which the monomeric surfactants self-assemble into thermodynamically stable aggregates is the CMC. The Krafft temperature of a surfactant is the lowest temperature required for micellization to take place. There are many parameters that affect the CMC. The interaction between the hydrophilic heads and the hydrophobic tails play a part, as well as the concentration of salt within the solution and surfactants.
Timothy P. Lodge is an American polymer scientist.
Interfacial rheology is a branch of rheology that studies the flow of matter at the interface between a gas and a liquid or at the interface between two immiscible liquids. The measurement is done while having surfactants, nanoparticles or other surface active compounds present at the interface. Unlike in bulk rheology, the deformation of the bulk phase is not of interest in interfacial rheology and its effect is aimed to be minimized. Instead, the flow of the surface active compounds is of interest..

Wetting solutions are liquids containing active chemical compounds that minimise the distance between two immiscible phases by lowering the surface tension to induce optimal spreading. The two phases, known as an interface, can be classified into five categories, namely, solid-solid, solid-liquid, solid-gas, liquid-liquid and liquid-gas.










