
Drosophila melanogaster is a species of fly in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly" or "pomace fly". Starting with Charles W. Woodworth's 1901 proposal of the use of this species as a model organism, D. melanogaster continues to be widely used for biological research in genetics, physiology, microbial pathogenesis, and life history evolution. As of 2017, five Nobel Prizes have been awarded to drosophilists for their work using the insect.

The human microbiome is the aggregate of all microbiota that reside on or within human tissues and biofluids along with the corresponding anatomical sites in which they reside, including the skin, mammary glands, seminal fluid, uterus, ovarian follicles, lung, saliva, oral mucosa, conjunctiva, biliary tract, and gastrointestinal tract. Types of human microbiota include bacteria, archaea, fungi, protists and viruses. Though micro-animals can also live on the human body, they are typically excluded from this definition. In the context of genomics, the term human microbiome is sometimes used to refer to the collective genomes of resident microorganisms; however, the term human metagenome has the same meaning.
Acetobacter is a genus of acetic acid bacteria. Acetic acid bacteria are characterized by the ability to convert ethanol to acetic acid in the presence of oxygen. Of these, the genus Acetobacter is distinguished by the ability to oxidize lactate and acetate into carbon dioxide and water. Bacteria of the genus Acetobacter have been isolated from industrial vinegar fermentation processes and are frequently used as fermentation starter cultures.
Gnotobiosis refers to an engineered state of an organism in which all forms of life in or on it, including its microbiota, have been identified. The term gnotobiotic organism, or gnotobiote, can refer to a model organism that is colonized with a specific community of known microorganisms or that contains no microorganisms (germ-free) often for experimental purposes. The study of gnotobiosis and the generation of various types of gnotobiotic model organisms as tools for studying interactions between host organisms and microorganisms is referred to as gnotobiology.

Gutmicrobiota are the microorganisms, including bacteria and archaea, that live in the digestive tracts of vertebrates including humans, and of insects. Alternative terms include gutflora and gutmicrobiome. The gastrointestinal metagenome is the aggregate of all the genomes of gut microbiota. In the human, the gut is the main location of human microbiota. The gut microbiota has broad impacts, including effects on colonization, resistance to pathogens, maintaining the intestinal epithelium, metabolizing dietary and pharmaceutical compounds, controlling immune function, and even behavior through the gut-brain axis.
Dysbiosis is characterized by a disruption to the microbiome resulting in an imbalance in the microbiota, changes in their functional composition and metabolic activities, or a shift in their local distribution. For example, a part of the human microbiota such as the skin flora, gut flora, or vaginal flora, can become deranged, with normally dominating species underrepresented and normally outcompeted or contained species increasing to fill the void. Dysbiosis is most commonly reported as a condition in the gastrointestinal tract, particularly during small intestinal bacterial overgrowth (SIBO) or small intestinal fungal overgrowth (SIFO).
Methanobrevibacter smithii is the predominant archaeon in the microbiota of the human gut. M. smithii has a coccobacillus shape. It plays an important role in the efficient digestion of polysaccharides by consuming the end products of bacterial fermentation. Methanobrevibacter smithii is a single-celled microorganism from the Archaea domain. M. smithii is a methanogen, and a hydrogenotroph that recycles the hydrogen by combining it with carbon dioxide to methane. The removal of hydrogen by M. smithii is thought to allow an increase in the extraction of energy from nutrients by shifting bacterial fermentation to more oxidized end products.
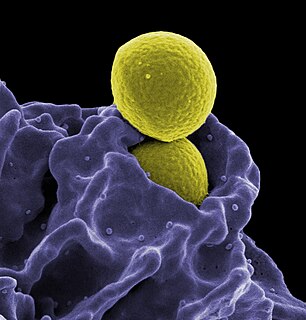
Long-term close-knit interactions between symbiotic microbes and their host can alter host immune system responses to other microorganisms, including pathogens, and are required to maintain proper homeostasis. The immune system is a host defense system consisting of anatomical physical barriers as well as physiological and cellular responses, which protect the host against harmful microorganisms while limiting host responses to harmless symbionts. Humans are home to 1013 to 1014 bacteria, roughly equivalent to the number of human cells, and while these bacteria can be pathogenic to their host most of them are mutually beneficial to both the host and bacteria.

Microbiota are the range of microorganisms that may be commensal, symbiotic, or pathogenic found in and on all multicellular organisms, including plants. Microbiota include bacteria, archaea, protists, fungi, and viruses, and have been found to be crucial for immunologic, hormonal, and metabolic homeostasis of their host.

The gut–brain axis is the two-way biochemical signaling that takes place between the gastrointestinal tract and the central nervous system (CNS). The term "gut–brain axis" is occasionally used to refer to the role of the gut microbiota in the interplay as well. The "microbiota–gut–brainaxis" explicitly includes the role of gut microbiota in the biochemical signaling events that take place between the GI tract and the CNS. Broadly defined, the gut–brain axis includes the central nervous system, neuroendocrine system, neuroimmune systems, the hypothalamic–pituitary–adrenal axis, sympathetic and parasympathetic arms of the autonomic nervous system, the enteric nervous system, vagus nerve, and the gut microbiota.
The altered Schaedler flora (ASF) is a community of eight bacterial species: two lactobacilli, one Bacteroides, one spiral bacterium of the Flexistipes genus, and four extremely oxygen sensitive (EOS) fusiform-shaped species. The bacteria are selected for their dominance and persistence in the normal microflora of mice, and for their ability to be isolated and grown in laboratory settings. Germ-free animals, mainly mice, are colonized with ASF for the purpose of studying the gastrointestinal (GI) tract. Intestinal mutualistic bacteria play an important role in affecting gene expression of the GI tract, immune responses, nutrient absorption, and pathogen resistance. The standardized microbial cocktail enabled the controlled study of microbe and host interactions, role of microbes, pathogen effects, and intestinal immunity and disease association, such as cancer, inflammatory bowel disease, diabetes, and other inflammatory or autoimmune diseases. Also, compared to germfree animals, ASF mice have fully developed immune system, resistance to opportunistic pathogens, and normal GI function and health, and are a great representation of normal mice.
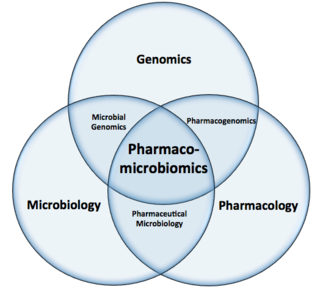
Pharmacomicrobiomics, first proposed by Prof. Marco Candela for the ERC-2009-StG project call and later publicly used in 2010, is defined as the effect of microbiome variations on drug disposition, action, and toxicity. Pharmacomicrobiomics is concerned with the interaction between xenobiotics, or foreign compounds, and the gut microbiome. It is estimated that over 100 trillion prokaryotes representing more than 1000 species reside in the gut. Within the gut, microbes help modulate developmental, immunological and nutrition host functions. The aggregate genome of microbes extends the metabolic capabilities of humans, allowing them to capture nutrients from diverse sources. Namely, through the secretion of enzymes that assist in the metabolism of chemicals foreign to the body, modification of liver and intestinal enzymes, and modulation of the expression of human metabolic genes, microbes can significantly impact the ingestion of xenobiotics.
The human milk microbiota, also known as human milk probiotics (HMP), refers to the microbiota residing in the human mammary glands and breast milk. Human breast milk has been traditionally assumed to be sterile, but more recently both microbial culture and culture-independent techniques have confirmed that human milk contains diverse communities of bacteria which are distinct from other microbial communities inhabiting the human body.
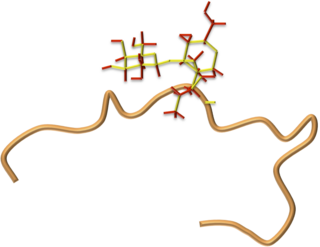
Drosocin is a 19-residue long antimicrobial peptide (AMP) of flies first isolated in the fruit fly Drosophila melanogaster, and later shown to be conserved throughout the genus Drosophila. Drosocin is regulated by the NF-κB Imd signalling pathway in the fly.

The Drosophila quinaria species group is a speciose lineage of mushroom-feeding flies studied for their specialist ecology, their parasites, population genetics, and the evolution of immune systems. Quinaria species are part of the Drosophila subgenus.

Drosophila innubila is a species of vinegar fly restricted to high-elevation woodlands in the mountains of the southern USA and Mexico, which it likely colonized during the last glacial period. Drosophila innubila is a kind of mushroom-breeding Drosophila, and member of the Drosophila quinaria species group. Drosophila innubila is best known for its association with a strain of male-killing Wolbachia bacteria. These bacteria are parasitic, as they drain resources from the host and cause half the infected female's eggs to abort. However Wolbachia may offer benefits to the fly's fitness in certain circumstances. The D. innubila genome was sequenced in 2019.
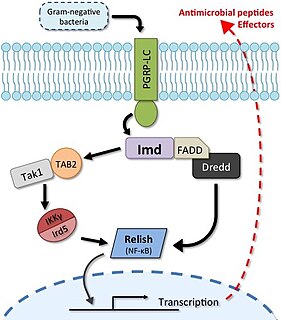
The Imd pathway is a broadly-conserved NF-κB immune signalling pathway of insects and some arthropods that regulates a potent antibacterial defence response. The pathway is named after the discovery of a mutation causing severe immune deficiency. The Imd pathway was first discovered in 1995 using Drosophila fruit flies by Bruno Lemaitre and colleagues, who also later discovered that the Drosophila Toll gene regulated defence against Gram-positive bacteria and fungi. Together the Toll and Imd pathways have formed a paradigm of insect immune signalling; as of September 2, 2019, these two landmark discovery papers have been cited collectively over 5000 times since publication on Google Scholar.

The Morganellaceae are a family of Gram-negative bacteria that include some important human pathogens formerly classified as Enterobacteriaceae. This family is a member of the order Enterobacterales in the class Gammaproteobacteria of the phylum Pseudomonadota. Genera in this family include the type genus Morganella, along with Arsenophonus, Cosenzaea, Moellerella, Photorhabdus, Proteus, Providencia and Xenorhabdus.

Bruno Lemaitre is a French immunologist and a professor at the École Polytechnique Fédérale de Lausanne (EPFL). His research focuses on the mechanisms of innate immunity and endosymbiosis in Drosophila. Lemaitre has also authored several books on the topic of narcissism in science.

Snodgrassella alvi is a species of Gram-negative bacteria within the Neisseriaceae and the only known species of the genus Snodgrassella. It was isolated and scientifically described in 2012 by Waldan K. Kwong and Nancy A. Moran, who named the bacteria after the American entomologist Robert Evans Snodgrass.













