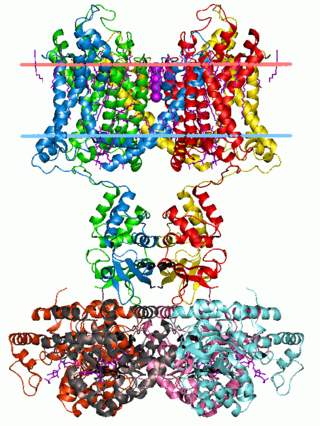
Potassium channels are the most widely distributed type of ion channel found in virtually all organisms. They form potassium-selective pores that span cell membranes. Potassium channels are found in most cell types and control a wide variety of cell functions.
Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and anti-inflammatory.
Sir Richard Peto is an English statistician and epidemiologist who is Professor of Medical Statistics and Epidemiology at the University of Oxford, England.

Taxanes are a class of diterpenes. They were originally identified from plants of the genus Taxus (yews), and feature a taxadiene core. Paclitaxel (Taxol) and docetaxel (Taxotere) are widely used as chemotherapy agents. Cabazitaxel was FDA approved to treat hormone-refractory prostate cancer.

Taxus canadensis, the Canada yew or Canadian yew, is a conifer native to central and eastern North America, thriving in swampy woods, ravines, riverbanks and on lake shores. Locally called simply as "yew", this species is also referred to as American yew or ground-hemlock.

Epothilones are a class of potential cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin, but in early trials, epothilones have better efficacy and milder adverse effects than taxanes.
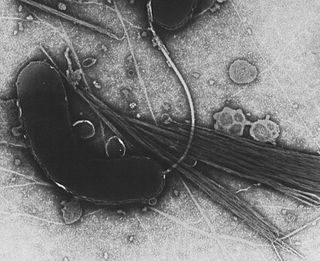
Gammaproteobacteria is a class of bacteria in the phylum Pseudomonadota. It contains about 250 genera, which makes it the most genus-rich taxon of the Prokaryotes. Several medically, ecologically, and scientifically important groups of bacteria belong to this class. All members of this class are Gram-negative. It is the most phylogenetically and physiologically diverse class of the Pseudomonadota.

Nicastrin, also known as NCSTN, is a protein that in humans is encoded by the NCSTN gene.

Ixabepilone is a pharmaceutical drug developed by Bristol-Myers Squibb as a chemotherapeutic medication for cancer.

Tubulin beta-3 chain, Class III β-tubulin, βIII-tubulin (β3-tubulin) or β-tubulin III, is a microtubule element of the tubulin family found almost exclusively in neurons, and in testis cells. In humans, it is encoded by the TUBB3 gene.

Retinoid X receptor alpha (RXR-alpha), also known as NR2B1 is a nuclear receptor that in humans is encoded by the RXRA gene.

Melanin-concentrating hormone receptor 1, also known as MCH1, is one of the melanin-concentrating hormone receptors found in all mammals.

CD3e molecule, epsilon also known as CD3E is a polypeptide which in humans is encoded by the CD3E gene which resides on chromosome 11.

Chloride anion exchanger, also known as down-regulated in adenoma, is a protein that in humans is encoded by the SLC26A3 gene.

Lysine-specific demethylase 5D is an enzyme that in humans is encoded by the KDM5D gene. KDM5D belongs to the alpha-ketoglutarate-dependent hydroxylases superfamily.

Activating transcription factor 5, also known as ATF5, is a protein that, in humans, is encoded by the ATF5 gene.

Sialidase-4 is an enzyme that in humans is encoded by the NEU4 gene.

Nucleoporin 205 (Nup205) is a protein that in humans is encoded by the NUP205 gene.
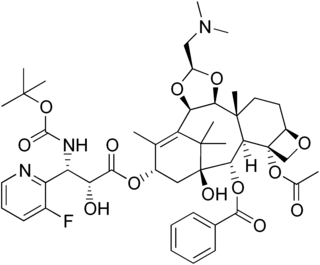
Tesetaxel is an orally administered taxane being investigated as a chemotherapy agent for various types of cancer, including breast cancer, gastric cancer, colorectal cancer, and other solid tumors. It differs from other members of the taxane class in that it is administered orally, not intravenously.

Teniloxazine, also known as sufoxazine and sulfoxazine, is a drug which is marketed in Japan. Though initially investigated as a neuroprotective and nootropic agent for the treatment of cerebrovascular insufficiency in the 1980s, it was ultimately developed and approved as an antidepressant instead. It acts as a potent norepinephrine reuptake inhibitor, with fair selectivity over the serotonin and dopamine transporters, and also behaves as an antagonist of the 5-HT2A receptor.

















