Related Research Articles
Hemiparesis, or unilateral paresis, is weakness of one entire side of the body. Hemiplegia is, in its most severe form, complete paralysis of half of the body. Hemiparesis and hemiplegia can be caused by different medical conditions, including congenital causes, trauma, tumors, or stroke.
A transient ischemic attack (TIA), commonly known as a mini-stroke, is a minor stroke whose noticeable symptoms usually end in less than an hour. TIA causes the same symptoms associated with strokes, such as weakness or numbness on one side of the body, sudden dimming or loss of vision, difficulty speaking or understanding language, slurred speech, or confusion.

An intracranial aneurysm, also known as a cerebral aneurysm, is a cerebrovascular disorder in which weakness in the wall of a cerebral artery or vein causes a localized dilation or ballooning of the blood vessel.
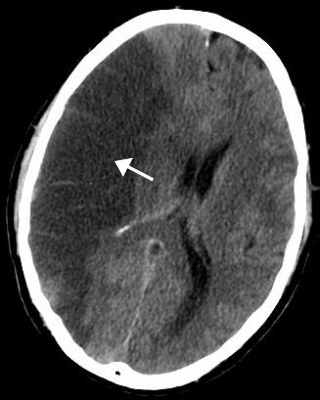
Stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functioning properly.
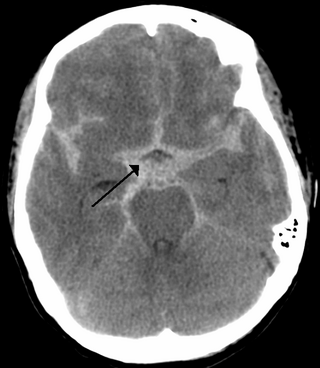
Subarachnoid hemorrhage (SAH) is bleeding into the subarachnoid space—the area between the arachnoid membrane and the pia mater surrounding the brain. Symptoms may include a severe headache of rapid onset, vomiting, decreased level of consciousness, fever, weakness, numbness, and sometimes seizures. Neck stiffness or neck pain are also relatively common. In about a quarter of people a small bleed with resolving symptoms occurs within a month of a larger bleed.
Alteplase, sold under the brand name Activase among others, is a biosynthetic form of human tissue-type plasminogen activator (t-PA). It is a thrombolytic medication used to treat acute ischemic stroke, acute ST-elevation myocardial infarction, pulmonary embolism associated with low blood pressure, and blocked central venous catheter. It is given by injection into a vein or artery. Alteplase is the same as the normal human plasminogen activator produced in vascular endothelial cells and is synthesized via recombinant DNA technology in Chinese hamster ovary cells (CHO). Alteplase causes the breakdown of a clot by inducing fibrinolysis.

Carotid artery stenosis is a narrowing or constriction of any part of the carotid arteries, usually caused by atherosclerosis.
A pain scale measures a patient's pain intensity or other features. Pain scales are a common communication tool in medical contexts, and are used in a variety of medical settings. Pain scales are a necessity to assist with better assessment of pain and patient screening. Pain measurements help determine the severity, type, and duration of the pain, and are used to make an accurate diagnosis, determine a treatment plan, and evaluate the effectiveness of treatment. Pain scales are based on trust, cartoons (behavioral), or imaginary data, and are available for neonates, infants, children, adolescents, adults, seniors, and persons whose communication is impaired. Pain assessments are often regarded as "the 5th vital sign".

Desmoteplase is a novel, highly fibrin-specific "clot-busting" (thrombolytic) drug in development that reached phase III clinical trials. The Danish pharmaceutical company, Lundbeck, owns the worldwide rights to Desmoteplase. In 2009, two large trials were started to test it as a safe and effective treatment for patients with acute ischaemic stroke. After disappointing results in DIAS-3, DIAS-4 was terminated, and in December 2014 Lundbeck announced that they would stop the development of desmoteplase.
Intracerebral hemorrhage (ICH), also known as hemorrhagic stroke, is a sudden bleeding into the tissues of the brain, into its ventricles, or into both. An ICH is a type of bleeding within the skull and one kind of stroke. Symptoms can vary dramatically depending on the severity, acuity, and location (anatomically) but can include headache, one-sided weakness, numbness, tingling, or paralysis, speech problems, vision or hearing problems, memory loss, attention problems, coordination problems, balance problems, dizziness or lightheadedness or vertigo, nausea/vomiting, seizures, decreased level of consciousness or total loss of consciousness, neck stiffness, and fever.

Cerebral infarction, also known as an ischemic stroke, is the pathologic process that results in an area of necrotic tissue in the brain. In mid to high income countries, a stroke is the main reason for disability among people and the 2nd cause of death. It is caused by disrupted blood supply (ischemia) and restricted oxygen supply (hypoxia). This is most commonly due to a thrombotic occlusion, or an embolic occlusion of major vessels which leads to a cerebral infarct. In response to ischemia, the brain degenerates by the process of liquefactive necrosis.

Vertebral artery dissection (VAD) is a flap-like tear of the inner lining of the vertebral artery, which is located in the neck and supplies blood to the brain. After the tear, blood enters the arterial wall and forms a blood clot, thickening the artery wall and often impeding blood flow. The symptoms of vertebral artery dissection include head and neck pain and intermittent or permanent stroke symptoms such as difficulty speaking, impaired coordination, and visual loss. It is usually diagnosed with a contrast-enhanced CT or MRI scan.
The Montgomery–Åsberg Depression Rating Scale (MADRS) is a ten-item diagnostic questionnaire which psychiatrists use to measure the severity of depressive episodes in patients with mood disorders. It was designed in 1979 by British and Swedish researchers as an adjunct to the Hamilton Rating Scale for Depression (HAMD) which would be more sensitive to the changes brought on by antidepressants and other forms of treatment than the Hamilton Scale was. There is, however, a high degree of statistical correlation between scores on the two measures.
The Barthel scale is an ordinal scale used to measure performance in activities of daily living (ADL). Each performance item is rated on this scale with a given number of points assigned to each level or ranking. It uses ten variables describing ADL and mobility. A higher number is associated with a greater likelihood of being able to live at home with a degree of independence following discharge from a hospital. The amount of time and physical assistance required to perform each item are used in determining the assigned value of each item. External factors within the environment affect the score of each item. If adaptations outside the standard home environment are met during assessment, the participant's score will be lower if these conditions are not available. If adaptations to the environment are made, they should be described in detail and attached to the Barthel index.
The National Institutes of Health Stroke Scale, or NIH Stroke Scale (NIHSS), is a tool used by healthcare providers to objectively quantify the impairment caused by a stroke and aid planning post-acute care disposition, though was intended to assess differences in interventions in clinical trials. The NIHSS was designed for the National Institute of Neurological Disorders and Stroke (NINDS) Recombinant Tissue Plasminogen Activator (rt-PA) for Acute Stroke Trial and was first published by neurologist Dr. Patrick Lyden and colleagues in 2001. Prior to the NIHSS, during the late 1980s, several stroke-deficit rating scales were in use.
A silent stroke is a stroke that does not have any outward symptoms associated with stroke, and the patient is typically unaware they have suffered a stroke. Despite not causing identifiable symptoms, a silent stroke still causes damage to the brain and places the patient at increased risk for both transient ischemic attack and major stroke in the future. In a broad study in 1998, more than 11 million people were estimated to have experienced a stroke in the United States. Approximately 770,000 of these strokes were symptomatic and 11 million were first-ever silent MRI infarcts or hemorrhages. Silent strokes typically cause lesions which are detected via the use of neuroimaging such as MRI. The risk of silent stroke increases with age but may also affect younger adults. Women appear to be at increased risk for silent stroke, with hypertension and current cigarette smoking being amongst the predisposing factors.
Neonatal stroke, similar to a stroke which occurs in adults, is defined as a disturbance to the blood supply of the developing brain in the first 28 days of life. This description includes both ischemic events, which results from a blockage of vessels, and hypoxic events, which results from a lack of oxygen to the brain tissue, as well as some combination of the two. One treatment with some proven benefits is hypothermia, but may be most beneficial in conjunction with pharmacological agents. Well-designed clinical trials for stroke treatment in neonates are lacking, but some current studies involve the transplantation of neural stem cells and umbilical cord stem cells; it is not yet known if this therapy is likely to be successful.
The Glasgow Outcome Scale (GOS) is an ordinal scale used to assess functional outcomes of patients following brain injury. It considers several factors, including a patient’s level of consciousness, ability to carry out activities of daily living (ADLs), and ability to return to work or school. The scale provides a structured way to classify patient outcomes into five broad categories: death, vegetative state, severe disability, moderate disability, or good recovery.
Embolic stroke of undetermined source (ESUS) is an embolic stroke, a type of ischemic stroke, with an unknown origin, defined as a non-lacunar brain infarct without proximal arterial stenosis or cardioembolic sources. As such, it forms a subset of cryptogenic stroke, which is part of the TOAST-classification. The following diagnostic criteria define an ESUS:
A cerebroprotectant is a drug that is intended to protect the brain after the onset of acute ischemic stroke. As stroke is the second largest cause of death worldwide and a leading cause of adult disability, over 150 drugs have been tested in clinical trials to provide cerebroprotection.
References
- 1 2 3 Wilson JL, Hareendran A, Grant M, et al. (2002). "Improving the Assessment of Outcomes in Stroke: Use of a Structured Interview to Assign Grades on the Modified Rankin Scale". Stroke. 33 (9): 2243–2246. doi: 10.1161/01.STR.0000027437.22450.BD . PMID 12215594.
- 1 2 Saver JL, Filip B, Hamilton S, et al. (2010). "Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA)". Stroke. 41 (5): 992–95. doi:10.1161/STROKEAHA.109.571364. PMC 2930146 . PMID 20360551.
- ↑ Quinn TJ, Dawson J, Walters M (2008). "Dr John Rankin; his life, legacy, and the 50th anniversary of the Rankin Stroke Scale". Scott Med J. 53 (1): 44–7. doi:10.1258/rsmsmj.53.1.44. PMID 18422210. S2CID 34909404.
- ↑ Rankin J (May 1957). "Cerebral vascular accidents in patients over the age of 60. II. Prognosis". Scott Med J. 2 (5): 200–15. doi:10.1177/003693305700200504. PMID 13432835. S2CID 29669359.
- ↑ van Swieten, J C; Koudstaal, P J; Visser, M C; Schouten, H J; van Gijn, J (May 1988). "Interobserver agreement for the assessment of handicap in stroke patients". Stroke. 19 (5): 604–607. doi: 10.1161/01.STR.19.5.604 . ISSN 0039-2499. PMID 3363593.
- ↑ Farrell B, Godwin J, Richards S, Warlow C, et al. (1991). "The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results". J Neurol Neurosurg Psychiatry. 54 (12): 1044–1054. doi:10.1136/jnnp.54.12.1044. PMC 1014676 . PMID 1783914.
- ↑ Wilson, J. T. Lindsay; Hareendran, Asha; Hendry, Anne; Potter, Jan; Bone, Ian; Muir, Keith W. (April 2005). "Reliability of the Modified Rankin Scale Across Multiple Raters: Benefits of a Structured Interview". Stroke. 36 (4): 777–781. doi: 10.1161/01.STR.0000157596.13234.95 . ISSN 0039-2499. PMID 15718510.
- 1 2 Wilson JL, Hareendran A, Hendry A, et al. (2005). "Reliability of the Modified Rankin Scale Across Multiple Raters: Benefits of a Structured Interview". Stroke. 36 (4): 777–781. doi: 10.1161/01.STR.0000157596.13234.95 . PMID 15718510.
- ↑ Quinn TJ, Lees KR, Hardemark HG, et al. (2007). "Initial experience of a digital training resource for modified Rankin scale assessment in clinical trials". Stroke. 38 (8): 2257–2261. doi: 10.1161/STROKEAHA.106.480723 . PMID 17600236.
- ↑ "Modified Rankin Scale for Neurologic Disability". MDCalc. Retrieved 2015-02-17.
- ↑ Bruno A, Shah N, Lin C, et al. (2010). "Improving modified Rankin Scale assessment with a simplified questionnaire". Stroke. 41 (5): 1048–50. doi: 10.1161/STROKEAHA.109.571562 . PMID 20224060.
- ↑ Patel N, Rao VA, Heilman-Espinoza ER, Lai R, Quesada RA, Flint AC (July 2012). "Simple and reliable determination of the modified Rankin Scale in neurosurgical and neurological patients: The mRS-9Q". Neurosurgery. 71 (5): 971–5, discussion 975. doi:10.1227/NEU.0b013e31826a8a56. PMID 22843133.