
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen or moisture slowly to form a black coating. Gadolinium below its Curie point of 20 °C (68 °F) is ferromagnetic, with an attraction to a magnetic field higher than that of nickel. Above this temperature it is the most paramagnetic element. It is found in nature only in an oxidized form. When separated, it usually has impurities of the other rare-earths because of their similar chemical properties.

A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.
In chemistry, solid-phase synthesis is a method in which molecules are covalently bound on a solid support material and synthesised step-by-step in a single reaction vessel utilising selective protecting group chemistry. Benefits compared with normal synthesis in a liquid state include:
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one fewer carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines.

Gadolinium(III) chloride, also known as gadolinium trichloride, is GdCl3. It is a colorless, hygroscopic, water-soluble solid. The hexahydrate GdCl3∙6H2O is commonly encountered and is sometimes also called gadolinium trichloride. Gd3+ species are of special interest because the ion has the maximum number of unpaired spins possible, at least for known elements. With seven valence electrons and seven available f-orbitals, all seven electrons are unpaired and symmetrically arranged around the metal. The high magnetism and high symmetry combine to make Gd3+ a useful component in NMR spectroscopy and MRI.

Contrast-enhanced ultrasound (CEUS) is the application of ultrasound contrast medium to traditional medical sonography. Ultrasound contrast agents rely on the different ways in which sound waves are reflected from interfaces between substances. This may be the surface of a small air bubble or a more complex structure. Commercially available contrast media are gas-filled microbubbles that are administered intravenously to the systemic circulation. Microbubbles have a high degree of echogenicity. There is a great difference in echogenicity between the gas in the microbubbles and the soft tissue surroundings of the body. Thus, ultrasonic imaging using microbubble contrast agents enhances the ultrasound backscatter, (reflection) of the ultrasound waves, to produce a sonogram with increased contrast due to the high echogenicity difference. Contrast-enhanced ultrasound can be used to image blood perfusion in organs, measure blood flow rate in the heart and other organs, and for other applications.

Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. Since this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl (Boc) group, it is commonly referred to as Boc anhydride. This pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. These carbamate derivatives do not behave as amines, which allows certain subsequent transformations to occur that would be incompatible with the amine functional group. The Boc group can later be removed from the amine using moderately strong acids. Thus, Boc serves as a protective group, for instance in solid phase peptide synthesis. Boc-protected amines are unreactive to most bases and nucleophiles, allowing for the use of the fluorenylmethyloxycarbonyl group (Fmoc) as an orthogonal protecting group.
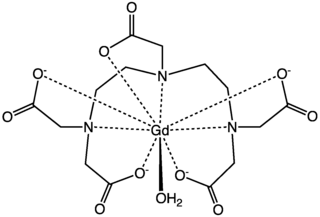
Gadopentetic acid, sold under the brand name Magnevist, is a gadolinium-based MRI contrast agent.

The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group is a protecting group used in organic synthesis.
Oseltamivir total synthesis concerns the total synthesis of the antiinfluenza drug oseltamivir marketed by Hoffmann-La Roche under the trade name Tamiflu. Its commercial production starts from the biomolecule shikimic acid harvested from Chinese star anise and from recombinant E. coli. Control of stereochemistry is important: the molecule has three stereocenters and the sought-after isomer is only 1 of 8 stereoisomers.
Bissulfosuccinimidyl suberate (BS3) is a crosslinker used in biological research. It is a water-soluble version of disuccinimidyl suberate.
MRI contrast agents are contrast agents used to improve the visibility of internal body structures in magnetic resonance imaging (MRI). The most commonly used compounds for contrast enhancement are gadolinium-based. Such MRI contrast agents shorten the relaxation times of nuclei within body tissues following oral or intravenous administration.

Ateviridine is a non-nucleoside reverse transcriptase inhibitor that has been studied for the treatment of HIV.

DOTA (also known as tetraxetan) is an organic compound with the formula (CH2CH2NCH2CO2H)4. The molecule consists of a central 12-membered tetraaza (i.e., containing four nitrogen atoms) ring. DOTA is used as a complexing agent, especially for lanthanide ions. Its complexes have medical applications as contrast agents and cancer treatments.

Disuccinimidyl suberate (DSS) is a six-carbon lysine-reactive non-cleavable cross-linking agent.

Biotin PEG2 amine is a water-soluble pegylated biotin derivative used as linker in biotechnology and molecular biology applications.

Perfusion MRI or perfusion-weighted imaging (PWI) is perfusion scanning by the use of a particular MRI sequence. The acquired data are then post-processed to obtain perfusion maps with different parameters, such as BV, BF, MTT and TTP.
Gadonanotube are carbon nanotubes containing superparamagnetic clusters of Gd3+ ions. They are linear molecular magnets and efficient contrast agents for magnetic resonance imaging (MRI). The term gadonanotube was introduced in 2005.














