Related Research Articles

Glycolysis is the metabolic pathway that converts glucose into pyruvate and, in most organisms, occurs in the liquid part of cells. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

Cellular respiration is the process by which biological fuels are oxidized in the presence of an inorganic electron acceptor, such as oxygen, to drive the bulk production of adenosine triphosphate (ATP), which contains energy. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into ATP, and then release waste products.
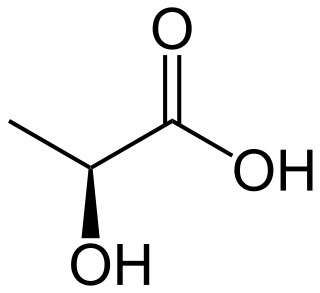
Lactic acid is an organic acid. It has the molecular formula CH3CH(OH)COOH. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate. The name of the derived acyl group is lactoyl.

Lactic acidosis is a medical condition characterized by a build-up of lactate in the body, with formation of an excessively low pH in the bloodstream. It is a form of metabolic acidosis, in which excessive acid accumulates due to a problem with the body's oxidative metabolism.

Tumor hypoxia is the situation where tumor cells have been deprived of oxygen. As a tumor grows, it rapidly outgrows its blood supply, leaving portions of the tumor with regions where the oxygen concentration is significantly lower than in healthy tissues. Hypoxic microenvironments in solid tumors are a result of available oxygen being consumed within 70 to 150 μm of tumor vasculature by rapidly proliferating tumor cells thus limiting the amount of oxygen available to diffuse further into the tumor tissue. In order to support continuous growth and proliferation in challenging hypoxic environments, cancer cells are found to alter their metabolism. Furthermore, hypoxia is known to change cell behavior and is associated with extracellular matrix remodeling and increased migratory and metastatic behavior.

The Cori cycle, named after its discoverers, Carl Ferdinand Cori and Gerty Cori, is a metabolic pathway in which lactate, produced by anaerobic glycolysis in muscles, is transported to the liver and converted to glucose, which then returns to the muscles and is cyclically metabolized back to lactate.
In oncology, the Warburg effect is the observation that most cancer cells release energy predominantly not through the 'usual' citric acid cycle and oxidative phosphorylation in the mitochondria as observed in normal cells, but through a less efficient process of 'anaerobic glycolysis' consisting of a high level of glucose uptake and glycolysis followed by lactic acid fermentation taking place in the cytosol, not the mitochondria, even in the presence of abundant oxygen. This observation was first published by Otto Heinrich Warburg, who was awarded the 1931 Nobel Prize in Physiology for his "discovery of the nature and mode of action of the respiratory enzyme". The precise mechanism and therapeutic implications of the Warburg effect, however, remain unclear.
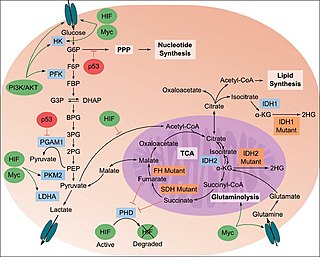
The study of the tumor metabolism, also known as tumor metabolome describes the different characteristic metabolic changes in tumor cells. The characteristic attributes of the tumor metabolome are high glycolytic enzyme activities, the expression of the pyruvate kinase isoenzyme type M2, increased channeling of glucose carbons into synthetic processes, such as nucleic acid, amino acid and phospholipid synthesis, a high rate of pyrimidine and purine de novo synthesis, a low ratio of Adenosine triphosphate and Guanosine triphosphate to Cytidine triphosphate and Uridine triphosphate, low Adenosine monophosphate levels, high glutaminolytic capacities, release of immunosuppressive substances and dependency on methionine.

The Warburg hypothesis, sometimes known as the Warburg theory of cancer, postulates that the driver of tumorigenesis is an insufficient cellular respiration caused by insult to mitochondria. The term Warburg effect in oncology describes the observation that cancer cells, and many cells grown in vitro, exhibit glucose fermentation even when enough oxygen is present to properly respire. In other words, instead of fully respiring in the presence of adequate oxygen, cancer cells ferment. The Warburg hypothesis was that the Warburg effect was the root cause of cancer. The current popular opinion is that cancer cells ferment glucose while keeping up the same level of respiration that was present before the process of carcinogenesis, and thus the Warburg effect would be defined as the observation that cancer cells exhibit glycolysis with lactate production and mitochondrial respiration even in the presence of oxygen.
The Pasteur effect describes how available oxygen inhibits ethanol fermentation, driving yeast to switch toward aerobic respiration for increased generation of the energy carrier adenosine triphosphate (ATP). More generally, in the medical literature, the Pasteur effect refers to how the cellular presence of oxygen causes in cells a decrease in the rate of glycolysis and also a suppression of lactate accumulation. The effect occurs in animal tissues, as well as in microorganisms belonging to the fungal kingdom.

Lactate dehydrogenase (LDH or LD) is an enzyme found in nearly all living cells. LDH catalyzes the conversion of pyruvate to lactate and back, as it converts NAD+ to NADH and back. A dehydrogenase is an enzyme that transfers a hydride from one molecule to another.

Monocarboxylate transporter 5 is a protein that in humans is encoded by the SLC16A4 gene.

Monocarboxylate transporter 4 (MCT4) also known as solute carrier family 16 member 3 is a protein that in humans is encoded by the SLC16A3 gene.

Pyruvate kinase isozymes M1/M2 (PKM1/M2), also known as pyruvate kinase muscle isozyme (PKM), pyruvate kinase type K, cytosolic thyroid hormone-binding protein (CTHBP), thyroid hormone-binding protein 1 (THBP1), or opa-interacting protein 3 (OIP3), is an enzyme that in humans is encoded by the PKM2 gene.

Monocarboxylate transporter 1 is a ubiquitous protein that in humans is encoded by the SLC16A1 gene. It is a proton coupled monocarboxylate transporter.

Inborn errors of carbohydrate metabolism are inborn error of metabolism that affect the catabolism and anabolism of carbohydrates.
The lactate shuttle hypothesis describes the movement of lactate intracellularly and intercellularly. The hypothesis is based on the observation that lactate is formed and utilized continuously in diverse cells under both anaerobic and aerobic conditions. Further, lactate produced at sites with high rates of glycolysis and glycogenolysis can be shuttled to adjacent or remote sites including heart or skeletal muscles where the lactate can be used as a gluconeogenic precursor or substrate for oxidation. The hypothesis was proposed 1985 by George Brooks of the University of California at Berkeley.

Monocarboxylate transporter 2 (MCT2) also known as solute carrier family 16 member 7 (SLC16A7) is a protein that in humans is encoded by the SLC16A7 gene. MCT2 is a proton-coupled monocarboxylate transporter. It catalyzes the rapid transport across the plasma membrane of many monocarboxylates such as lactic acid, branched-chain oxo acids derived from [[leucine, valine, and isoleucine, and the ketone bodies acetoacetate and beta-hydroxybutyrate. It also functions as high-affinity pyruvate transporter.

Monocarboxylate transporter 3 (MCT3) also known as solute carrier family 16 member 8 is a protein that in humans is encoded by the SLC16A8 gene. MCT is a proton-coupled monocarboxylate transporter. It catalyzes the rapid transport across the plasma membrane of many monocarboxylates such as lactate, pyruvate, branched-chain oxo acids derived from leucine, valine and isoleucine, and the ketone bodies acetoacetate, beta-hydroxybutyrate and acetate. It also functions as high-affinity pyruvate transporter.
Aerobic fermentation or aerobic glycolysis is a metabolic process by which cells metabolize sugars via fermentation in the presence of oxygen and occurs through the repression of normal respiratory metabolism. Preference of aerobic fermentation over aerobic respiration is referred to as the Crabtree effect in yeast, and is part of the Warburg effect in tumor cells. While aerobic fermentation does not produce adenosine triphosphate (ATP) in high yield, it allows proliferating cells to convert nutrients such as glucose and glutamine more efficiently into biomass by avoiding unnecessary catabolic oxidation of such nutrients into carbon dioxide, preserving carbon-carbon bonds and promoting anabolism.
References
- ↑ Halestrap AP, Meredith D (2004). "The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond". Pflügers Arch. 447 (5): 619–28. doi:10.1007/s00424-003-1067-2. PMID 12739169. S2CID 15498611.
- 1 2 3 4 Felmlee MA, Jones RS, Morris ME (2020). "Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease". Pharmacological Reviews . 72 (2): 466–485. doi:10.1124/pr.119.018762. PMC 7062045 . PMID 32144120.
- ↑ Jugder, Bat-Erdene; Kamareddine, Layla; Watnick, Paula I. (August 2021). "Microbiota-derived acetate activates intestinal innate immunity via the Tip60 histone acetyltransferase complex". Immunity. doi:10.1016/j.immuni.2021.05.017. PMC 8363570 .
- ↑ Parks, Scott K.; Mueller-Klieser, Wolfgang; Pouysségur, Jacques (2020). "Lactate and Acidity in the Cancer Microenvironment". Annual Review of Cancer Biology. 4: 141–158. doi: 10.1146/annurev-cancerbio-030419-033556 .
- ↑ Lupton, H. (1923). "An analysis of the effects of speed on the mechanical efficiency of human muscular movement". J Physiol. 57 (6): 337–53. doi:10.1113/jphysiol.1923.sp002072. PMC 1405479 . PMID 16993578.
- ↑ Alfarouk, KO; Shayoub, ME; Muddathir, AK; Elhassan, GO; Bashir, AH (22 July 2011). "Evolution of Tumor Metabolism might Reflect Carcinogenesis as a Reverse Evolution process (Dismantling of Multicellularity)". Cancers. 3 (4): 3002–17. doi: 10.3390/cancers3033002 . PMC 3759183 . PMID 24310356.
- ↑ Mathupala SP, Colen CB, Parajuli P, Sloan AE (2007). "Lactate and malignant tumors: a therapeutic target at the end stage of glycolysis (Review)". J Bioenerg Biomembr. 39 (1): 73–77. doi:10.1007/s10863-006-9062-x. PMC 3385854 . PMID 17354062.
- ↑ Mathupala SP, Parajuli P, Sloan AE (2004). "Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study". Neurosurgery. 55 (6): 1410–1419. doi:10.1227/01.neu.0000143034.62913.59. PMID 15574223.
- ↑ Colen, CB, PhD Thesis (2005) http://elibrary.wayne.edu/record=b3043899~S47
- ↑ Colen CB, Seraji-Bozorgzad N, Marples B, Galloway MP, Sloan AE, Mathupala SP (2006). "Metabolic remodeling of malignant gliomas for enhanced sensitization during radiotherapy: an in vitro study". Neurosurgery. 59 (6): 1313–1323. doi:10.1227/01.NEU.0000249218.65332.BF. PMC 3385862 . PMID 17277695.
- ↑ Colen CB, Shen Y, Ghoddoussi F, Yu P, Francis TB, Koch BJ, Monterey MD, Galloway MP, Sloan AE, Mathupala SP (2011). "Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study". Neoplasia. 13 (7): 620–632. doi:10.1593/neo.11134. PMC 3132848 . PMID 21750656.