Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agent. It is used as a water cleaner and as an etchant for metals.
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is now being appreciated, siderophores are among the strongest (highest affinity) Fe3+ binding agents known. Phytosiderophores are siderophores produced by plants.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.

In organic chemistry, hydroxamic acids are a class of organic compounds bearing the functional group R−C(=O)−N(OH)−R', with R and R' as organic residues. They are amides wherein the nitrogen center has a hydroxyl substituent. They are often used as metal chelators.

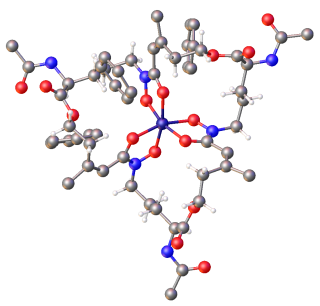
Enterobactin is a high affinity siderophore that acquires iron for microbial systems. It is primarily found in Gram-negative bacteria, such as Escherichia coli and Salmonella typhimurium.

Kenneth Norman Raymond is a bioinorganic and coordination chemist. He is Chancellor's Professor of Chemistry at the University of California, Berkeley, Professor of the Graduate School, the Director of the Seaborg Center in the Chemical Sciences Division at Lawrence Berkeley National Laboratory, and the President and Chairman of Lumiphore.
Chiral Lewis acids (CLAs) are a type of Lewis acid catalyst. These acids affect the chirality of the substrate as they react with it. In such reactions, synthesis favors the formation of a specific enantiomer or diastereomer. The method is an enantioselective asymmetric synthesis reaction. Since they affect chirality, they produce optically active products from optically inactive or mixed starting materials. This type of preferential formation of one enantiomer or diastereomer over the other is formally known as asymmetric induction. In this kind of Lewis acid, the electron-accepting atom is typically a metal, such as indium, zinc, lithium, aluminium, titanium, or boron. The chiral-altering ligands employed for synthesizing these acids often have multiple Lewis basic sites that allow the formation of a ring structure involving the metal atom.

Ferrichrome is a cyclic hexa-peptide that forms a complex with iron atoms. It is a siderophore composed of three glycine and three modified ornithine residues with hydroxamate groups [-N(OH)C(=O)C-]. The 6 oxygen atoms from the three hydroxamate groups bind Fe(III) in near perfect octahedral coordination.

Thujaplicins are a series of tropolone-related chemical substances that have been isolated from the softwoods of the trees of Cupressaceae family. These compounds are known for their antibacterial, antifungal, and antioxidant properties. They were the first natural tropolones to be made synthetically.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early and late d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen, nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.
Siderocalin(Scn), lipocalin-2, NGAL, 24p3 is a mammalian lipocalin-type protein that can prevent iron acquisition by pathogenic bacteria by binding siderophores, which are iron-binding chelators made by microorganisms. Iron serves as a key nutrient in host-pathogen interactions, and pathogens can acquire iron from the host organism via synthesis and release siderophores such as enterobactin. Siderocalin is a part of the mammalian defence mechanism and acts as an antibacterial agent. Crystallographic studies of Scn demonstrated that it includes a calyx, a ligand-binding domain that is lined with polar cationic groups. Central to the siderophore/siderocalin recognition mechanism are hybrid electrostatic/cation-pi interactions. To evade the host defences, pathogens evolved to produce structurally varied siderophores that would not be recognized by siderocalin, allowing the bacteria to acquire iron.

Rebecca Abergel is a French inorganic chemist who specializes in the coordination chemistry between lanthanide and actinide complexes. Alongside the effects of heavy element exposure and contamination on different biological systems. Abergel is currently a faculty scientist and heavy element chemistry group leader at the chemical sciences division of Lawrence Berkeley National Laboratory in Berkeley, California. She is also assistant professor of nuclear engineering at University of California, Berkeley.

Transition metal thioether complexes comprise coordination complexes of thioether (R2S) ligands. The inventory is extensive.

Transition metal carboxylate complexes are coordination complexes with carboxylate (RCO2−) ligands. Reflecting the diversity of carboxylic acids, the inventory of metal carboxylates is large. Many are useful commercially, and many have attracted intense scholarly scrutiny. Carboxylates exhibit a variety of coordination modes, most common are κ1- (O-monodentate), κ2 (O,O-bidentate), and bridging.

Rhizoferrin is an organic compound with the formula (CH2CH2NHCOCH2C(OH)(CO2H)CH2CO2H)2. It is multifunctional molecule with two secondary alcohols, four carboxylic acid groups, and two amide groups. In aqueous solution, it is highly ionized, but the term rhizoferrin is still applied to these species.

Petrobactin is a bis-catechol siderophore produced by M. hydrocarbonoclasticus, A. macleodii, and the anthrax-producing B. anthracis. Like other siderophores petrobactin is a highly specific iron(III) transport ligand, contributing to the marine microbial uptake of environmental iron.














