
The Belgian Blue is a breed of beef cattle from Belgium. It may also be known as the Race de la Moyenne et Haute Belgique, or dikbil. Alternative names for this breed include Belgian Blue-White; Belgian White and Blue Pied; Belgian White Blue; Blue; and Blue Belgian. The Belgian Blue's extremely lean, hyper-sculpted, ultra-muscular physique is termed "double-muscling". The double-muscling phenotype is a heritable condition resulting in an increased number of muscle fibres (hyperplasia), instead of the (normal) enlargement of individual muscle fibres (hypertrophy).
Barth syndrome (BTHS) is a rare but serious X-linked genetic disorder, caused by changes in phospholipid structure and metabolism. It may affect multiple body systems, and is potentially fatal. The syndrome is diagnosed almost exclusively in males.

Limb–girdle muscular dystrophy (LGMD) is a genetically heterogeneous group of rare muscular dystrophies that share a set of clinical characteristics. It is characterised by progressive muscle wasting which affects predominantly hip and shoulder muscles. LGMD usually has an autosomal pattern of inheritance. It currently has no known cure or treatment.

Myostatin is a protein that in humans is encoded by the MSTN gene. Myostatin is a myokine that is produced and released by myocytes and acts on muscle cells to inhibit muscle growth. Myostatin is a secreted growth differentiation factor that is a member of the TGF beta protein family.
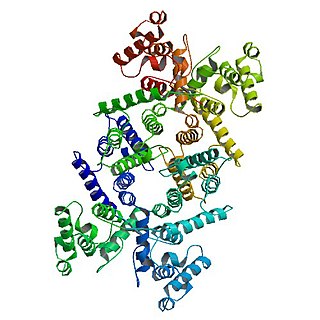
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex (DAPC). Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere. It has a molecular weight of 427 kDa

Duchenne muscular dystrophy (DMD) is a severe type of muscular dystrophy that primarily affects boys. Muscle weakness usually begins around the age of four, and worsens quickly. Muscle loss typically occurs first in the thighs and pelvis followed by the arms. This can result in trouble standing up. Most are unable to walk by the age of 12. Affected muscles may look larger due to increased fat content. Scoliosis is also common. Some may have intellectual disability. Females with a single copy of the defective gene may show mild symptoms.

CRISPR is a family of DNA sequences found in the genomes of prokaryotic organisms such as bacteria and archaea. These sequences are derived from DNA fragments of bacteriophages that had previously infected the prokaryote. They are used to detect and destroy DNA from similar bacteriophages during subsequent infections. Hence these sequences play a key role in the antiviral defense system of prokaryotes and provide a form of acquired immunity. CRISPR is found in approximately 50% of sequenced bacterial genomes and nearly 90% of sequenced archaea.

Follistatin also known as activin-binding protein is a protein that in humans is encoded by the FST gene. Follistatin is an autocrine glycoprotein that is expressed in nearly all tissues of higher animals.

Myogenesis is the formation of skeletal muscular tissue, particularly during embryonic development.

Muscle hypertrophy or muscle building involves a hypertrophy or increase in size of skeletal muscle through a growth in size of its component cells. Two factors contribute to hypertrophy: sarcoplasmic hypertrophy, which focuses more on increased muscle glycogen storage; and myofibrillar hypertrophy, which focuses more on increased myofibril size. It is the primary focus of bodybuilding-related activities.

Growth differentiation factor 11 (GDF11) also known as bone morphogenetic protein 11 (BMP-11) is a protein that in humans is encoded by the growth differentiation factor 11 gene. GDF11 is a member of the Transforming growth factor beta family.
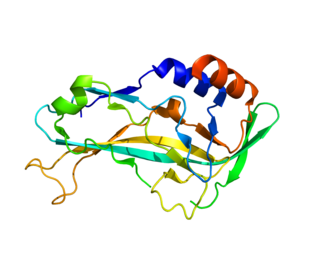
T-box transcription factor TBX5, is a protein that in humans is encoded by the TBX5 gene. Abnormalities in the TBX5 gene can result in altered limb development, Holt-Oram syndrome, Tetra-amelia syndrome, and cardiac and skeletal problems.
Double-muscled cattle refers to breeds of cattle that carry one of seven known mutations that limits and reduces the activity of the myostatin protein. Normally, myostatin limits the number of muscle fibers present at birth, and interfering with activity of this protein causes animals to be born with higher numbers of muscle fibers, consequently augmenting muscle growth. Additionally, these mutations reduce the superficial and internal fat deposits, causing the meat to be less marbled and lower in fat content. Animals homozygous for myostatin mutation also have improved meat tenderness in some cuts of meat. The enlarged muscles of dam and calf at birth leads to increased difficulty of calving, and in some breeds frequently necessitates birth by cesarean section.

Genetically modified mammals are mammals that have been genetically engineered. They are an important category of genetically modified organisms. The majority of research involving genetically modified mammals involves mice with attempts to produce knockout animals in other mammalian species limited by the inability to derive and stably culture embryonic stem cells.
A myokine is one of several hundred cytokines or other small proteins and proteoglycan peptides that are produced and released by skeletal muscle cells in response to muscular contractions. They have autocrine, paracrine and/or endocrine effects; their systemic effects occur at picomolar concentrations.

Genome editing, or genome engineering, or gene editing, is a type of genetic engineering in which DNA is inserted, deleted, modified or replaced in the genome of a living organism. Unlike early genetic engineering techniques that randomly inserts genetic material into a host genome, genome editing targets the insertions to site-specific locations. The basic mechanism involved in genetic manipulations through programmable nucleases is the recognition of target genomic loci and binding of effector DNA-binding domain (DBD), double-strand breaks (DSBs) in target DNA by the restriction endonucleases, and the repair of DSBs through homology-directed recombination (HDR) or non-homologous end joining (NHEJ).
Human germline engineering is the process by which the genome of an individual is edited in such a way that the change is heritable. This is achieved through genetic alterations within the germ cells, or the reproductive cells, such as the egg and sperm. Human germline engineering is a type of genetic modification that directly manipulates the genome using molecular engineering techniques. Aside from germline engineering, genetic modification can be applied in another way, somatic genetic modification. Somatic gene modification consists of altering somatic cells, which are all cells in the body that are not involved in reproduction. While somatic gene therapy does change the genome of the targeted cells, these cells are not within the germline, so the alterations are not heritable and cannot be passed on to the next generation.
Off-target genome editing refers to nonspecific and unintended genetic modifications that can arise through the use of engineered nuclease technologies such as: clustered, regularly interspaced, short palindromic repeats (CRISPR)-Cas9, transcription activator-like effector nucleases (TALEN), meganucleases, and zinc finger nucleases (ZFN). These tools use different mechanisms to bind a predetermined sequence of DNA (“target”), which they cleave, creating a double-stranded chromosomal break (DSB) that summons the cell's DNA repair mechanisms and leads to site-specific modifications. If these complexes do not bind at the target, often a result of homologous sequences and/or mismatch tolerance, they will cleave off-target DSB and cause non-specific genetic modifications. Specifically, off-target effects consist of unintended point mutations, deletions, insertions inversions, and translocations.

CRISPR gene editing is a genetic engineering technique in molecular biology by which the genomes of living organisms may be modified. It is based on a simplified version of the bacterial CRISPR-Cas9 antiviral defense system. By delivering the Cas9 nuclease complexed with a synthetic guide RNA (gRNA) into a cell, the cell's genome can be cut at a desired location, allowing existing genes to be removed and/or new ones added in vivo.
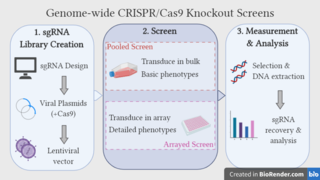
Genome-wide CRISPR-Cas9 knockout screens aim to elucidate the relationship between genotype and phenotype by ablating gene expression on a genome-wide scale and studying the resulting phenotypic alterations. The approach utilises the CRISPR-Cas9 gene editing system, coupled with libraries of single guide RNAs (sgRNAs), which are designed to target every gene in the genome. Over recent years, the genome-wide CRISPR screen has emerged as a powerful tool for performing large-scale loss-of-function screens, with low noise, high knockout efficiency and minimal off-target effects.














