A retrovirus is a type of virus that inserts a DNA copy of its RNA genome into the DNA of a host cell that it invades, thus changing the genome of that cell. After invading a host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backward). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus. Many retroviruses cause serious diseases in humans, other mammals, and birds.

Myxomatosis is a disease caused by Myxoma virus, a poxvirus in the genus Leporipoxvirus. The natural hosts are tapeti in South and Central America, and brush rabbits in North America. The myxoma virus causes only a mild disease in these species, but causes a severe and usually fatal disease in European rabbits, the species of rabbit commonly raised for companionship and as a food source.
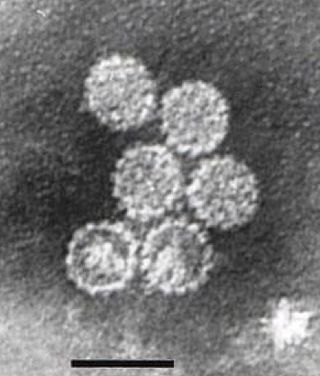
Papillomaviridae is a family of non-enveloped DNA viruses whose members are known as papillomaviruses. Several hundred species of papillomaviruses, traditionally referred to as "types", have been identified infecting all carefully inspected mammals, but also other vertebrates such as birds, snakes, turtles and fish. Infection by most papillomavirus types, depending on the type, is either asymptomatic or causes small benign tumors, known as papillomas or warts. Papillomas caused by some types, however, such as human papillomaviruses 16 and 18, carry a risk of becoming cancerous.
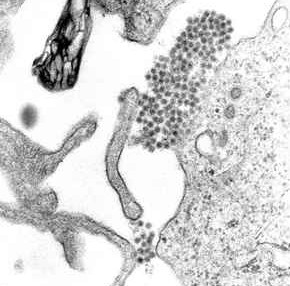
Dengue virus (DENV) is the cause of dengue fever. It is a mosquito-borne, single positive-stranded RNA virus of the family Flaviviridae; genus Flavivirus. Four serotypes of the virus have been found, and a reported fifth has yet to be confirmed, all of which can cause the full spectrum of disease. Nevertheless, the mainstream scientific community's understanding of dengue virus may be simplistic as, rather than distinct antigenic groups, a continuum appears to exist. This same study identified 47 strains of dengue virus. Additionally, coinfection with and lack of rapid tests for Zika virus and chikungunya complicate matters in real-world infections.

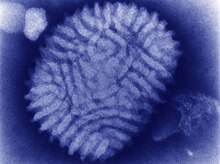
The measles virus (MV), with scientific name Morbillivirus hominis, is a single-stranded, negative-sense, enveloped, non-segmented RNA virus of the genus Morbillivirus within the family Paramyxoviridae. It is the cause of measles. Humans are the natural hosts of the virus; no animal reservoirs are known to exist.

Lassa mammarenavirus (LASV) is an arenavirus that causes Lassa hemorrhagic fever, a type of viral hemorrhagic fever (VHF), in humans and other primates. Lassa mammarenavirus is an emerging virus and a select agent, requiring Biosafety Level 4-equivalent containment. It is endemic in West African countries, especially Sierra Leone, the Republic of Guinea, Nigeria, and Liberia, where the annual incidence of infection is between 300,000 and 500,000 cases, resulting in 5,000 deaths per year.
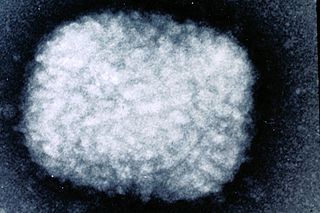
Poxviridae is a family of double-stranded DNA viruses. Vertebrates and arthropods serve as natural hosts. There are currently 83 species in this family, divided among 22 genera, which are divided into two subfamilies Chordopoxvirinae and Entomopoxvirinae. Entomopoxvirinae infect insects and Chordopoxvirinae infect vertebrates. Diseases associated with this family include smallpox.
Viral pathogenesis is the study of the process and mechanisms by which viruses cause diseases in their target hosts, often at the cellular or molecular level. It is a specialized field of study in virology.

Polydnaviriformidae ( PDV) is a family of insect viriforms; members are known as polydnaviruses. There are two genera in the family: Bracoform and Ichnoviriform. Polydnaviruses form a symbiotic relationship with parasitoid wasps. Ichnoviriforms (IV) occur in Ichneumonid wasps and Bracoviriforms (BV) in Braconid wasps. The larvae of wasps in both of those groups are themselves parasitic on Lepidoptera, and the polydnaviruses are important in circumventing the immune response of their parasitized hosts. Little or no sequence homology exists between BV and IV, suggesting that the two genera have been evolving independently for a long time.

Gammaretrovirus is a genus in the Retroviridae family. Example species are the murine leukemia virus and the feline leukemia virus. They cause various sarcomas, leukemias and immune deficiencies in mammals, reptiles and birds.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.

A viral envelope is the outermost layer of many types of viruses. It protects the genetic material in their life cycle when traveling between host cells. Not all viruses have envelopes. A viral envelope protein or E protein is a protein in the envelope, which may be acquired by the capsid from an infected host cell.

Molluscum contagiosum virus (MCV) is a species of DNA poxvirus that causes the human skin infection molluscum contagiosum. Molluscum contagiosum affects about 200,000 people a year, about 1% of all diagnosed skin diseases. Diagnosis is based on the size and shape of the skin lesions and can be confirmed with a biopsy, as the virus cannot be routinely cultured. Molluscum contagiosum virus is the only species in the genus Molluscipoxvirus. MCV is a member of the subfamily Chordopoxvirinae of family Poxviridae. Other commonly known viruses that reside in the subfamily Chordopoxvirinae are variola virus and monkeypox virus.
Drosophila X virus (DXV) belongs to the Birnaviridae family of viruses. Birnaviridae currently consists of three genera. The first genus is Entomobirnavirus, which contains DXV. The next genus is Aquabirnavirus, containing infectious pancreatic necrosis virus (IPNV). The last genus is Avibirnavirus, which contains infectious bursal disease virus (IBDV). All of these genera contain homology in three specific areas of their transcripts. The homology comes from the amino and carboxyl regions of preVP2, a small 21-residue-long domain near the carboxyl terminal of VP3, and similar small ORFs sequences.

Mengovirus, also known as Columbia SK virus, mouse Elberfield virus, and Encephalomyocarditis virus (EMCV), belongs to the genus Cardiovirus which is a member of the Picornaviridae. Its genome is a single stranded positive-sense RNA molecule, making the Mengoviruses a class IV virus under the Baltimore classification system. The genome is approximately 8400nt in length, and has 5’ VG protein and a 3’ polyadenine tail. Mengovirus was isolated by George W. A. Dick in 1948, in the Mengo district of Entebbe in Uganda, from a captive rhesus monkey that had developed hind limb paralysis.

Viral shedding is the expulsion and release of virus progeny following successful reproduction during a host cell infection. Once replication has been completed and the host cell is exhausted of all resources in making viral progeny, the viruses may begin to leave the cell by several methods.
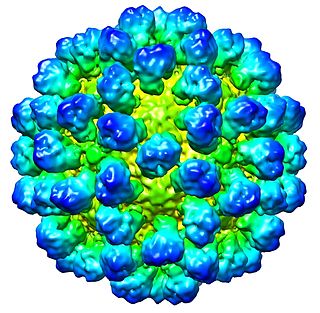
Lagovirus is a genus of viruses, in the family Caliciviridae. Lagomorphs serve as natural hosts. There are two species in this genus. Diseases associated with this genus include: necrotizing hepatitis leading to fatal hemorrhages.
Leporipoxvirus is a genus of viruses, in the family Poxviridae, in the subfamily Chordopoxvirinae. Lagomorphs and squirrels serve as natural hosts. There are four species in this genus. Diseases associated with this genus include: myxomatosis.
This glossary of virology is a list of definitions of terms and concepts used in virology, the study of viruses, particularly in the description of viruses and their actions. Related fields include microbiology, molecular biology, and genetics.
Sepik virus (SEPV) is an arthropod-borne virus (arbovirus) of the genus Flavivirus and family Flaviviridae. Flaviviridae is one of the most well characterized viral families, as it contains many well-known viruses that cause diseases that have become very prevalent in the world, like Dengue virus. The genus Flavivirus is one of the largest viral genera and encompasses over 50 viral species, including tick and mosquito borne viruses like Yellow fever virus and West Nile virus. Sepik virus is much less well known and has not been as well-classified as other viruses because it has not been known of for very long. Sepik virus was first isolated in 1966 from the mosquito Mansoniaseptempunctata, and it derives its name from the Sepik River area in Papua New Guinea, where it was first found. The geographic range of Sepik virus is limited to Papua New Guinea, due to its isolation.