
Imperial Chemical Industries (ICI) was a British chemical company. It was, for much of its history, the largest manufacturer in Britain. It was formed by the merger of four leading British chemical companies in 1926. Its headquarters were at Millbank in London. ICI was a constituent of the FT 30 and later the FTSE 100 indices.

William Nunn Lipscomb Jr. was a Nobel Prize-winning American inorganic and organic chemist working in nuclear magnetic resonance, theoretical chemistry, boron chemistry, and biochemistry.

Rayon is a semi-synthetic fiber, made from natural sources of regenerated cellulose, such as wood and related agricultural products. It has the same molecular structure as cellulose. It is also called viscose. Many types and grades of viscose fibers and films exist. Some imitate the feel and texture of natural fibers such as silk, wool, cotton, and linen. The types that resemble silk are often called artificial silk.
Twaron is a para-aramid. It is a heat-resistant and strong synthetic fibre developed in the early 1970s by the Dutch company Akzo Nobel's division Enka BV, later Akzo Industrial Fibers. The research name of the para-aramid fibre was originally Fiber X, but it was soon called Arenka. Although the Dutch para-aramid fiber was developed only a little later than DuPont's Kevlar, the introduction of Twaron as a commercial product came much later than Kevlar due to financial problems at the AKZO company in the 1970s.

Akzo Nobel N.V., stylized as AkzoNobel, is a Dutch multinational company which creates paints and performance coatings for both industry and consumers worldwide. Headquartered in Amsterdam, the company has activities in more than 80 countries, and employs over 32,000 people. Sales in 2020 were €8.5 billion.

In organic chemistry, a Schiff base is a compound with the general structure R1R2C=NR3. They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimines depending on their structure. The term is often synonymous with azomethine which refers specifically to secondary aldimines.

PPG Industries, Inc. is an American Fortune 500 company and global supplier of paints, coatings, and specialty materials. With headquarters in Pittsburgh, Pennsylvania, PPG operates in more than 70 countries around the globe. By revenue it is the largest coatings company in the world followed by AkzoNobel. It is headquartered in PPG Place, an office and retail complex in downtown Pittsburgh, and is known for its glass facade designed by Postmodern architect Philip Johnson.

Safranin is a biological stain used in histology and cytology. Safranin is used as a counterstain in some staining protocols, colouring cell nuclei red. This is the classic counterstain in both Gram stains and endospore staining. It can also be used for the detection of cartilage, mucin and mast cell granules.

Cucurbiturils are macrocyclic molecules made of glycoluril monomers linked by methylene bridges. The oxygen atoms are located along the edges of the band and are tilted inwards, forming a partly enclosed cavity. The name is derived from the resemblance of this molecule with a pumpkin of the family of Cucurbitaceae.
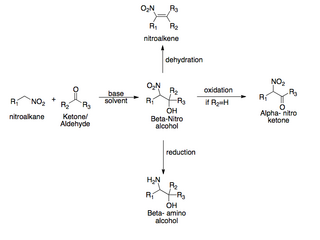
The Henry reaction is a classic carbon–carbon bond formation reaction in organic chemistry. Discovered in 1895 by the Belgian chemist Louis Henry (1834–1913), it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-nitro alcohols. This type of reaction is also referred to as a nitroaldol reaction. It is nearly analogous to the aldol reaction that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols". The Henry reaction is a useful technique in the area of organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols.
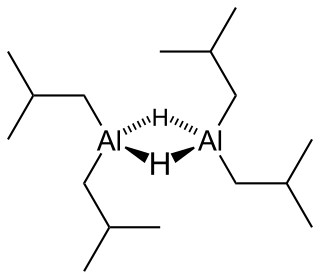
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (i-Bu2AlH)2, where i-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound is a reagent in organic synthesis.
Trypan blue is an azo dye. It is a direct dye for cotton textiles. In biosciences, it is used as a vital stain to selectively colour dead tissues or cells blue.
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term diamine refers mostly to primary diamines, as those are the most reactive.
In chemistry, the Noyori asymmetric hydrogenation refers to methodology for enantioselective reduction of ketones and related functional groups. This methodology was introduced by Ryoji Noyori, who shared the Nobel Prize in Chemistry in 2001 for contributions to asymmetric hydrogenation. These hydrogenations are used in the production of several drugs, such as the antibacterial levofloxin, the antibiotic carbapenem, and the antipsychotic agent BMS181100.
Organon & Co. is an American pharmaceutical company headquartered in Jersey City, New Jersey. Organon specializes in the following core therapeutic fields: reproductive medicine, contraception, psychiatry, hormone replacement therapy (HRT), and anesthesia. Organon sells to international markets.
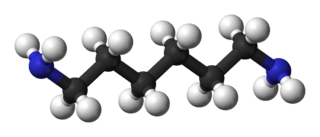
Hexamethylenediamine is the organic compound with the formula H2N(CH2)6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid (yellowish for some commercial samples) has a strong amine odor. About 1 billion kilograms are produced annually.

Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand. It is used as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform prochiral alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.

1,2-Diaminopropane (propane-1,2-diamine) is organic compound with the formula CH3CH(NH2)CH2NH2. A colorless liquid, it is the simplest chiral diamine. It is used as a bidentate ligand in coordination chemistry.
The Bargellini reaction is a chemical reaction discovered in 1906 by Italian chemist Guido Bargellini. The original reaction was a mixture of the reagents phenol, chloroform, and acetone in the presence of a sodium hydroxide solution. Prior to Bargellini's research, the product attributed to this multi-component reaction (MCR) had been described as a phenol derivative in chemistry texts at the time. However, Bargellini demonstrated that a carboxylic acid derivative was actually the correct structure.

The nitro-Mannich reaction is the nucleophilic addition of a nitroalkane to an imine, resulting in the formation of a beta-nitroamine. With the reaction involving the addition of an acidic carbon nucleophile to a carbon-heteroatom double bond, the nitro-Mannich reaction is related to some of the most fundamental carbon-carbon bond forming reactions in organic chemistry, including the aldol reaction, Henry reaction and Mannich reaction.




















