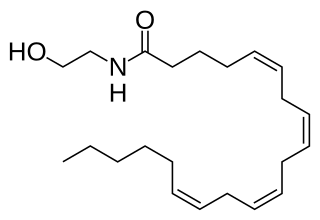
Anandamide (ANA), also known as N-arachidonoylethanolamine (AEA), is a fatty acid neurotransmitter. Anandamide was the first endocannabinoid to be discovered: it participates in the body's endocannabinoid system by binding to cannabinoid receptors, the same receptors that the psychoactive compound THC in cannabis acts on. Anandamide is found in nearly all tissues in a wide range of animals. Anandamide has also been found in plants, including small amounts in chocolate. The name 'anandamide' is taken from the Sanskrit word ananda, which means "joy, bliss, delight", and amide.

URB597 (KDS-4103) is a relatively selective and irreversible inhibitor of the enzyme fatty acid amide hydrolase (FAAH). FAAH is the primary degradatory enzyme for the endocannabinoid anandamide and, as such, inhibition of FAAH leads to an accumulation of anandamide in the CNS and periphery where it activates cannabinoid receptors. URB597 has been found to elevate anandamide levels and have activity against neuropathic pain in a mouse model.

Monoacylglycerol lipase, also known as MAG lipase, acylglycerol lipase, MAGL, MGL or MGLL is an enzyme that, in humans, is encoded by the MGLL gene. MAGL is a 33-kDa, membrane-associated member of the serine hydrolase superfamily and contains the classical GXSXG consensus sequence common to most serine hydrolases. The catalytic triad has been identified as Ser122, His269, and Asp239.

Fatty acid amide hydrolase or FAAH is a member of the serine hydrolase family of enzymes. It was first shown to break down anandamide in 1993. In humans, it is encoded by the gene FAAH.

AM404, also known as N-arachidonoylaminophenol, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.

Methoxy arachidonyl fluorophosphonate, commonly referred as MAFP, is an irreversible active site-directed enzyme inhibitor that inhibits nearly all serine hydrolases and serine proteases. It inhibits phospholipase A2 and fatty acid amide hydrolase with special potency, displaying IC50 values in the low-nanomolar range. In addition, it binds to the CB1 receptor in rat brain membrane preparations (IC50 = 20 nM), but does not appear to agonize or antagonize the receptor, though some related derivatives do show cannabinoid-like properties.

In enzymology, an amidase is an enzyme that catalyzes the hydrolysis of an amide:

N-acylethanolamine-hydrolyzing acid amidase is an enzyme that in humans is encoded by the NAAA gene.
N-Acylphosphatidylethanolamines (NAPEs) are hormones released by the small intestine into the bloodstream when it processes fat. NAPEs travel to the hypothalamus in the brain and suppress appetite. This mechanism could be relevant for treating obesity.
N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) is an enzyme that catalyzes the release of N-acylethanolamine (NAE) from N-acyl-phosphatidylethanolamine (NAPE). This is a major part of the process that converts ordinary lipids into chemical signals like anandamide and oleoylethanolamine. In humans, the NAPE-PLD protein is encoded by the NAPEPLD gene.

An N-acylethanolamine (NAE) is a type of fatty acid amide formed when one of several types of acyl group is linked to the nitrogen atom of ethanolamine. These amides conceptually can be formed from a fatty acid and ethanolamine with the release of a molecule of water, but the known biological synthesis uses a specific phospholipase D to cleave the phospholipid unit from N-acylphosphatidylethanolamines. Another route relies on the transesterification of acyl groups from phosphatidylcholine by an N-acyltransferase (NAT) activity. The suffixes -amine and -amide in these names each refer to the single nitrogen atom of ethanolamine that links the compound together: it is termed "amine" in ethanolamine because it is considered as a free terminal nitrogen in that subunit, while it is termed "amide" when it is considered in association with the adjacent carbonyl group of the acyl subunit. Names for these compounds may be encountered with either "amide" or "amine" varying by author.
Palmitoylethanolamide (PEA) is an endogenous fatty acid amide, belonging to the class of nuclear factor agonists. PEA has been studied in in vitro and in vivo systems using exogenously added or dosed compound; there is evidence that it binds to a nuclear receptor, through which it exerts a variety of biological effects, some related to chronic inflammation and pain.

JZL195 is a potent inhibitor of both fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), the primary enzymes responsible for degrading the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG), respectively.

LY-2183240 is a drug which acts both as a potent inhibitor of the reuptake of the endocannabinoid anandamide and as an inhibitor of fatty acid amide hydrolase (FAAH), the primary enzyme responsible for degrading anandamide. This leads to markedly elevated anandamide levels in the brain, and LY-2183240 has been shown to produce both analgesic and anxiolytic effects in animal models. While LY-2183240 is a potent inhibitor of FAAH, it has relatively poor selectivity and also inhibits several other enzyme side targets. Consequently, it was never developed for clinical use, though it remains widely used in research, and has also been sold as a designer drug.
PF-04457845 is a potent and exquisitely selective inhibitor of the enzyme fatty acid amide hydrolase (FAAH), with an IC50 of 7.2nM, and both analgesic and antiinflammatory effects in animal studies comparable to naproxen.
IDFP is an organophosphorus compound related to the nerve agent sarin.
The endocannabinoid transporters (eCBTs) are transport proteins for the endocannabinoids. Most neurotransmitters are water-soluble and require transmembrane proteins to transport them across the cell membrane. The endocannabinoids on the other hand, are non-charged lipids that readily cross lipid membranes. However, since the endocannabinoids are water immiscible, protein transporters have been described that act as carriers to solubilize and transport the endocannabinoids through the aqueous cytoplasm. These include the heat shock proteins (Hsp70s) and fatty acid-binding proteins for anandamide (FABPs). FABPs such as FABP1, FABP3, FABP5, and FABP7 have been shown to bind endocannabinoids. FABP inhibitors attenuate the breakdown of anandamide by the enzyme fatty acid amide hydrolase (FAAH) in cell culture. One of these inhibitors (SB-FI-26), isolated from a virtual library of a million compounds, belongs to a class of compounds that act as an anti-nociceptive agent with mild anti-inflammatory activity in mice. These truxillic acids and their derivatives have been known to have anti-inflammatory and anti-nociceptive effects in mice and are active components of a Chinese herbal medicine used to treat rheumatism and pain in human. The blockade of anandamide transport may, at least in part, be the mechanism through which these compounds exert their anti-nociceptive effects.

N-acyl amides are a general class of endogenous fatty acid compounds characterized by a fatty acyl group linked to a primary amine metabolite by an amide bond. Broadly speaking, N-acyl amides fall into several categories: amino acid conjugates, neurotransmitter conjugates, ethanolamine conjugates, and taurine conjugates. N-acyl amides have pleiotropic signaling functions in physiology, including in cardiovascular function, metabolic homeostasis, memory, cognition, pain, motor control and others. Initial attention focused on N-acyl amides present in mammalian organisms, however recently lipid signaling systems consisting of N-acyl amides have also been found to be present in invertebrates, such as Drosophila melanogaster. N-acyl amides play important roles in many biochemical pathways involved in a variety of physiological and pathological processes, as well as the metabolic enzymes, transporters, and receptors that regulate their signaling.
PF-3845 is a selective inhibitor of fatty acid amide hydrolase. It results in increased levels of anandamide and results in cannabinoid receptor-based effects. It has anti-inflammatory action in mice colitis models. Antidiarrheal and antinociceptive effects were also seen in mouse models of pain.

Fatty acid amide hydrolase 2 or FAAH2 is a member of the serine hydrolase family of enzymes.














