
Pemetrexed, sold under the brand name Alimta among others, is a chemotherapy medication for the treatment of pleural mesothelioma and non-small cell lung cancer (NSCLC)..
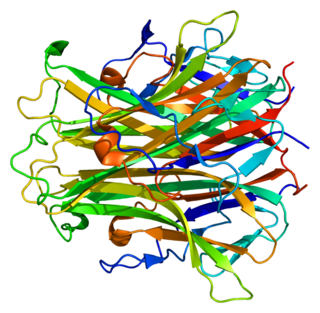
Receptor activator of nuclear factor kappa-Β ligand (RANKL), also known as tumor necrosis factor ligand superfamily member 11 (TNFSF11), TNF-related activation-induced cytokine (TRANCE), osteoprotegerin ligand (OPGL), and osteoclast differentiation factor (ODF), is a protein that in humans is encoded by the TNFSF11 gene.
Coramsine (SBP002) was an experimental cancer drug that was evaluated in preliminary clinical trials, but was abandoned by Solbec Pharmaceuticals Ltd after the results were insufficient for them to raise investment capital to continue its development.

Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
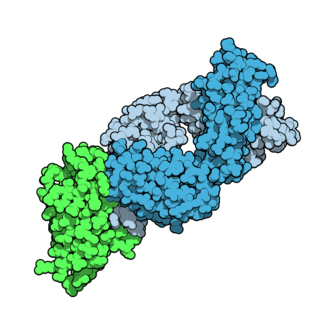
Tremelimumab, sold under the brand name Imjudo, is a fully human monoclonal antibody used for the treatment of hepatocellular carcinoma. Tremelimumab is designed to attach to and block CTLA-4, a protein that controls the activity of T cells, which are part of the immune system.
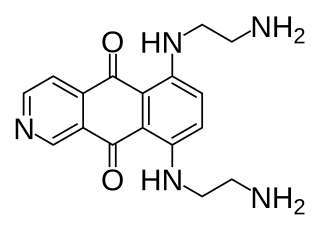
Pixantrone is an experimental antineoplastic (anti-cancer) drug, an analogue of mitoxantrone with fewer toxic effects on cardiac tissue. It acts as a topoisomerase II poison and intercalating agent. The code name BBR 2778 refers to pixantrone dimaleate, the actual substance commonly used in clinical trials.
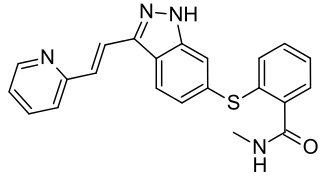
Axitinib, sold under the brand name Inlyta, is a small molecule tyrosine kinase inhibitor developed by Pfizer. It has been shown to significantly inhibit growth of breast cancer in animal (xenograft) models and has shown partial responses in clinical trials with renal cell carcinoma (RCC) and several other tumour types.
Electrochemotherapy (ECT) is a type of chemotherapy that allows delivery of non-permeant drugs to the cell interior. It is based on the local application of short and intense electric pulses that transiently permeabilize the cell membrane, thus allowing transport of molecules otherwise not permitted by the membrane. Applications for treatment of cutaneous and subcutaneous tumors have reached clinical use by utilizing drugs such as bleomycin or cisplatin). Electrochemotherapy with bleomycin was used to treat a patient for the first time in 1991 at the Institute Gustave Roussy in France, while electrochemotherapy with cisplatin was used to treat for the first time in 1995 at the Institute of Oncology, Ljubljana, Slovenia. Since then, more than 4000 patients were treated with electrochemotherapy all over the world. Recently, new electrochemotherapy modalities have been developed for treatment of internal tumors using surgical procedures, endoscopic routes, or percutaneous approaches to gain access to the treatment area.

Eribulin, sold under the brand name Halaven, is an anticancer medication used to treat breast cancer and liposarcoma.
Ramucirumab is a fully human monoclonal antibody (IgG1) developed for the treatment of solid tumors. This drug was developed by ImClone Systems Inc. It was isolated from a native phage display library from Dyax.

Romidepsin, sold under the brand name Istodax, is an anticancer agent used in cutaneous T-cell lymphoma (CTCL) and other peripheral T-cell lymphomas (PTCLs). Romidepsin is a natural product obtained from the bacterium Chromobacterium violaceum, and works by blocking enzymes known as histone deacetylases, thus inducing apoptosis. It is sometimes referred to as depsipeptide, after the class of molecules to which it belongs. Romidepsin is branded and owned by Gloucester Pharmaceuticals, a part of Celgene.
Folate targeting is a method utilized in biotechnology for drug delivery purposes. This Trojan Horse process, which was created by Drs. Christopher P. Leamon and Philip S. Low, involves the attachment of the vitamin, folate, to a molecule/drug to form a "folate conjugate". Based on the natural high affinity of folate for the folate receptor protein (FR), which is commonly expressed on the surface of many human cancers, folate-drug conjugates also bind tightly to the FR and trigger cellular uptake via endocytosis. Molecules as diverse as small radiodiagnostic imaging agents to large DNA plasmid formulations have successfully been delivered inside FR-positive cells and tissues.
Brentuximab vedotin, sold under the brand name Adcetris, is an antibody-drug conjugate medication used to treat relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL), a type of T cell non-Hodgkin lymphoma. It selectively targets tumor cells expressing the CD30 antigen, a defining marker of Hodgkin lymphoma and ALCL. The drug is being jointly marketed by Millennium Pharmaceuticals outside the US and by Seagen in the US.

Crizotinib, sold under the brand name Xalkori among others, is an anti-cancer medication used for the treatment of non-small cell lung carcinoma (NSCLC). It acts as an ALK and ROS1 inhibitor.
Edelfosine is a synthetic alkyl-lysophospholipid (ALP). It has antineoplastic (anti-cancer) effects.
Angiokinase inhibitors are a new therapeutic target for the management of cancer. They inhibit tumour angiogenesis, one of the key processes leading to invasion and metastasis of solid tumours, by targeting receptor tyrosine kinases. Examples include nintedanib, afatinib and motesanib.
Amatuximab is a chimeric monoclonal antibody designed for the treatment of cancer. It was developed by Morphotek, Inc.
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.

Lurbinectedin, sold under the brand name Zepzelca, is a medication used for the treatment of small cell lung cancer.
Cytokines are polypeptides or glycoproteins that help immune cells communicate to each other to induce proliferation, activation, differentiation, and inflammatory or anti-inflammatory signals in various cell types. Studies utilizing cytokines for antitumor therapies has increased significantly since 2000, and different cytokines provide unique antitumor activities. Cytokines hinder tumor cell development mostly through antiproliferative or proapoptotic pathways but can also interrupt development indirectly by eliciting immune cells to have cytotoxic effects against tumor cells. Even though there are FDA-approved cytokine therapies, there are two main challenges associated with cytokine delivery. The first is that cytokines have a short half-life, so frequent administration of high doses is required for therapeutic effect. The second is that systemic toxicity could occur if the cytokines delivered cause an intense immune response, known as a cytokine storm.









