
Spirulina is a biomass of cyanobacteria that can be consumed by humans and animals. The three species are Arthrospira platensis, A. fusiformis, and A. maxima.

Microalgae or microphytes are microscopic algae invisible to the naked eye. They are phytoplankton typically found in freshwater and marine systems, living in both the water column and sediment. They are unicellular species which exist individually, or in chains or groups. Depending on the species, their sizes can range from a few micrometers (μm) to a few hundred micrometers. Unlike higher plants, microalgae do not have roots, stems, or leaves. They are specially adapted to an environment dominated by viscous forces.

Algaculture is a form of aquaculture involving the farming of species of algae.
Pyrolysis oil, sometimes also known as bio-crude or bio-oil, is a synthetic fuel under investigation as substitute for petroleum. It is obtained by heating dried biomass without oxygen in a reactor at a temperature of about 500 °C (900 °F) with subsequent cooling. Pyrolysis oil is a kind of tar and normally contains levels of oxygen too high to be considered a pure hydrocarbon. This high oxygen content results in non-volatility, corrosiveness, immiscibility with fossil fuels, thermal instability, and a tendency to polymerize when exposed to air. As such, it is distinctly different from petroleum products. Removing oxygen from bio-oil or nitrogen from algal bio-oil is known as upgrading.

A photobioreactor (PBR) refers to any cultivation system designed for growing photoautotrophic organisms using artificial light sources or solar light to facilitate photosynthesis. PBRs are typically used to cultivate microalgae, cyanobacteria, and some mosses. PBRs can be open systems, such as raceway ponds, which rely upon natural sources of light and carbon dioxide. Closed PBRs are flexible systems that can be controlled to the physiological requirements of the cultured organism, resulting in optimal growth rates and purity levels. PBRs are typically used for the cultivation of bioactive compounds for biofuels, pharmaceuticals, and other industrial uses.
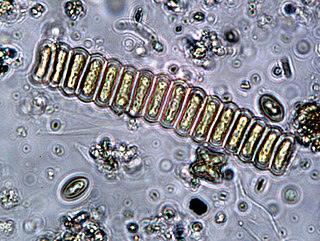
Scenedesmus is a genus of green algae, in the class Chlorophyceae. They are colonial and non-motile. They are one of the most common components of phytoplankton in freshwater habitats worldwide.
Auxenochlorella protothecoides, formerly known as Chlorella protothecoides, is a facultative heterotrophic green alga in the family Chlorellaceae. It is known for its potential application in biofuel production. It was first characterized as a distinct algal species in 1965, and has since been regarded as a separate genus from Chlorella due its need for thiamine for growth. Auxenochlorella species have been found in a wide variety of environments from acidic volcanic soil in Italy to the sap of poplar trees in the forests of Germany. Its use in industrial processes has been studied, as the high lipid content of the alga during heterotrophic growth is promising for biodiesel; its use in wastewater treatment has been investigated, as well.

Choricystis is a genus of green algae in the class Trebouxiophyceae, considered a characteristic picophytoplankton in freshwater ecosystems. Choricystis, especially the type species Choricystis minor, has been proposed as an effective source of fatty acids for biofuels. Choricystis algacultures have been shown to survive on wastewater. In particular, Choricystis has been proposed as a biological water treatment system for industrial waste produced by the processing of dairy goods.

Algae fuel, algal biofuel, or algal oil is an alternative to liquid fossil fuels that uses algae as its source of energy-rich oils. Also, algae fuels are an alternative to commonly known biofuel sources, such as corn and sugarcane. When made from seaweed (macroalgae) it can be known as seaweed fuel or seaweed oil.

Nannochloropsis is a genus of algae comprising six known species. The genus in the current taxonomic classification was first termed by Hibberd (1981). The species have mostly been known from the marine environment but also occur in fresh and brackish water. All of the species are small, nonmotile spheres which do not express any distinct morphological features that can be distinguished by either light or electron microscopy. The characterisation is mostly done by rbcL gene and 18S rRNA sequence analysis.
An algae bioreactor is used for cultivating micro or macroalgae. Algae may be cultivated for the purposes of biomass production (as in a seaweed cultivator), wastewater treatment, CO2 fixation, or aquarium/pond filtration in the form of an algae scrubber. Algae bioreactors vary widely in design, falling broadly into two categories: open reactors and enclosed reactors. Open reactors are exposed to the atmosphere while enclosed reactors, also commonly called photobioreactors, are isolated to varying extents from the atmosphere. Specifically, algae bioreactors can be used to produce fuels such as biodiesel and bioethanol, to generate animal feed, or to reduce pollutants such as NOx and CO2 in flue gases of power plants. Fundamentally, this kind of bioreactor is based on the photosynthetic reaction, which is performed by the chlorophyll-containing algae itself using dissolved carbon dioxide and sunlight. The carbon dioxide is dispersed into the reactor fluid to make it accessible to the algae. The bioreactor has to be made out of transparent material.
Nasrin Moazami is an Iranian medical microbiologist and biotechnologist. She received her Ph.D. in 1976 from the Faculty of Medicine at Laval University. Moazami is the pioneer of biotechnology and microalgae-based fuels in Iran.

Microalgae or microscopic algae grow in either marine or freshwater systems. They are primary producers in the oceans that convert water and carbon dioxide to biomass and oxygen in the presence of sunlight.
Single cell oil, also known as Microbial oil consists of the intracellular storage lipids, triacyglycerols. It is similar to vegetable oil, another biologically produced oil. They are produced by oleaginous microorganisms, which is the term for those bacteria, molds, algae and yeast, which can accumulate 20% to 80% lipids of their biomass. The accumulation of lipids take place by the end of logarithmic phase and continues during station phase until carbon source begins to reduce with nutrition limitation.
Schizochytrium is a genus of unicellular eukaryotes in the family Thraustochytriaceae, which are found in coastal marine habitats. They are assigned to the Stramenopiles (heterokonts), a group which also contains kelp and various microalgae.

Avigad Vonshak is a Professor Emeritus at the French Associates Institute for Agriculture and Biotechnology of Drylands at the Jacob Blaustein Institutes for Desert Research at Ben-Gurion University of the Negev, Israel.
Sammy Boussiba is a professor emeritus at the French Associates Institute for Agriculture and Biotechnology of Drylands at the Jacob Blaustein Institutes for Desert Research at Ben-Gurion University of the Negev, Israel.

Chlorella vulgaris is a species of green microalga in the division Chlorophyta. It is mainly used as a dietary supplement or protein-rich food additive in Japan.
Algal viruses are the viruses infecting photosynthetic single-celled eukaryotes, algae. As of 2020, there were 61 viruses known to infect algae. Algae are integral components of aquatic food webs and drive nutrient cycling, so the viruses infecting algal populations also impacts the organisms and nutrient cycling systems that depend on them. Thus, these viruses can have significant, worldwide economic and ecological effects. Their genomes varied between 4.4 to 560 kilobase pairs (kbp) long and used double-stranded Deoxyribonucleic Acid (dsDNA), double-stranded Ribonucleic Acid (dsRNA), single-stranded Deoxyribonucleic Acid (ssDNA), and single-stranded Ribonucleic Acid (ssRNA). The viruses ranged between 20 and 210 nm in diameter. Since the discovery of the first algae-infecting virus in 1979, several different techniques have been used to find new viruses infecting algae and it seems that there are many algae-infecting viruses left to be discovered












