Nanomedicine is the medical application of nanotechnology. Nanomedicine ranges from the medical applications of nanomaterials and biological devices, to nanoelectronic biosensors, and even possible future applications of molecular nanotechnology such as biological machines. Current problems for nanomedicine involve understanding the issues related to toxicity and environmental impact of nanoscale materials.

A liposome is a small artificial vesicle, spherical in shape, having at least one lipid bilayer. Due to their hydrophobicity and/or hydrophilicity, biocompatibility, particle size and many other properties, liposomes can be used as drug delivery vehicles for administration of pharmaceutical drugs and nutrients, such as lipid nanoparticles in mRNA vaccines, and DNA vaccines. Liposomes can be prepared by disrupting biological membranes.
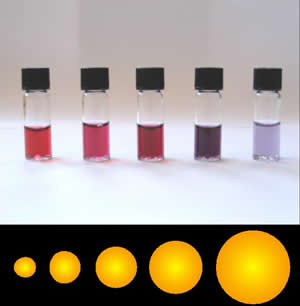
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red or blue-purple . Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine.

Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably.
A drug carrier or drug vehicle is a substrate used in the process of drug delivery which serves to improve the selectivity, effectiveness, and/or safety of drug administration. Drug carriers are primarily used to control the release of drugs into systemic circulation. This can be accomplished either by slow release of a particular drug over a long period of time or by triggered release at the drug's target by some stimulus, such as changes in pH, application of heat, and activation by light. Drug carriers are also used to improve the pharmacokinetic properties, specifically the bioavailability, of many drugs with poor water solubility and/or membrane permeability.
Targeted drug delivery, sometimes called smart drug delivery, is a method of delivering medication to a patient in a manner that increases the concentration of the medication in some parts of the body relative to others. This means of delivery is largely founded on nanomedicine, which plans to employ nanoparticle-mediated drug delivery in order to combat the downfalls of conventional drug delivery. These nanoparticles would be loaded with drugs and targeted to specific parts of the body where there is solely diseased tissue, thereby avoiding interaction with healthy tissue. The goal of a targeted drug delivery system is to prolong, localize, target and have a protected drug interaction with the diseased tissue. The conventional drug delivery system is the absorption of the drug across a biological membrane, whereas the targeted release system releases the drug in a dosage form. The advantages to the targeted release system is the reduction in the frequency of the dosages taken by the patient, having a more uniform effect of the drug, reduction of drug side-effects, and reduced fluctuation in circulating drug levels. The disadvantage of the system is high cost, which makes productivity more difficult, and the reduced ability to adjust the dosages.

Lipid nanoparticles (LNPs) are nanoparticles composed of lipids. They are a novel pharmaceutical drug delivery system, and a novel pharmaceutical formulation. LNPs as a drug delivery vehicle were first approved in 2018 for the siRNA drug Onpattro. LNPs became more widely known in late 2020, as some COVID-19 vaccines that use RNA vaccine technology coat the fragile mRNA strands with PEGylated lipid nanoparticles as their delivery vehicle.

A nanoparticle–biomolecule conjugate is a nanoparticle with biomolecules attached to its surface. Nanoparticles are minuscule particles, typically measured in nanometers (nm), that are used in nanobiotechnology to explore the functions of biomolecules. Properties of the ultrafine particles are characterized by the components on their surfaces more so than larger structures, such as cells, due to large surface area-to-volume ratios. Large surface area-to-volume-ratios of nanoparticles optimize the potential for interactions with biomolecules.

Gene therapy utilizes the delivery of DNA into cells, which can be accomplished by several methods, summarized below. The two major classes of methods are those that use recombinant viruses and those that use naked DNA or DNA complexes.

A nanocarrier is nanomaterial being used as a transport module for another substance, such as a drug. Commonly used nanocarriers include micelles, polymers, carbon-based materials, liposomes and other substances. Nanocarriers are currently being studied for their use in drug delivery and their unique characteristics demonstrate potential use in chemotherapy. This class of materials was first reported by a team of researchers of University of Évora, Alentejo in early 1960's, and grew exponentially in relevance since then.
A nanocapsule is a nanoscale shell made from a nontoxic polymer. They are vesicular systems made of a polymeric membrane which encapsulates an inner liquid core at the nanoscale. Nanocapsules have many uses, including promising medical applications for drug delivery, food enhancement, nutraceuticals, and for self-healing materials. The benefits of encapsulation methods are for protection of these substances to protect in the adverse environment, for controlled release, and for precision targeting. Nanocapsules can potentially be used as MRI-guided nanorobots or nanobots, although challenges remain.
Nanoparticles for drug delivery to the brain is a method for transporting drug molecules across the blood–brain barrier (BBB) using nanoparticles. These drugs cross the BBB and deliver pharmaceuticals to the brain for therapeutic treatment of neurological disorders. These disorders include Parkinson's disease, Alzheimer's disease, schizophrenia, depression, and brain tumors. Part of the difficulty in finding cures for these central nervous system (CNS) disorders is that there is yet no truly efficient delivery method for drugs to cross the BBB. Antibiotics, antineoplastic agents, and a variety of CNS-active drugs, especially neuropeptides, are a few examples of molecules that cannot pass the BBB alone. With the aid of nanoparticle delivery systems, however, studies have shown that some drugs can now cross the BBB, and even exhibit lower toxicity and decrease adverse effects throughout the body. Toxicity is an important concept for pharmacology because high toxicity levels in the body could be detrimental to the patient by affecting other organs and disrupting their function. Further, the BBB is not the only physiological barrier for drug delivery to the brain. Other biological factors influence how drugs are transported throughout the body and how they target specific locations for action. Some of these pathophysiological factors include blood flow alterations, edema and increased intracranial pressure, metabolic perturbations, and altered gene expression and protein synthesis. Though there exist many obstacles that make developing a robust delivery system difficult, nanoparticles provide a promising mechanism for drug transport to the CNS.
Poly(amidoamine), or PAMAM, is a class of dendrimer which is made of repetitively branched subunits of amide and amine functionality. PAMAM dendrimers, sometimes referred to by the trade name Starburst, have been extensively studied since their synthesis in 1985, and represent the most well-characterized dendrimer family as well as the first to be commercialized. Like other dendrimers, PAMAMs have a sphere-like shape overall, and are typified by an internal molecular architecture consisting of tree-like branching, with each outward 'layer', or generation, containing exponentially more branching points. This branched architecture distinguishes PAMAMs and other dendrimers from traditional polymers, as it allows for low polydispersity and a high level of structural control during synthesis, and gives rise to a large number of surface sites relative to the total molecular volume. Moreover, PAMAM dendrimers exhibit greater biocompatibility than other dendrimer families, perhaps due to the combination of surface amines and interior amide bonds; these bonding motifs are highly reminiscent of innate biological chemistry and endow PAMAM dendrimers with properties similar to that of globular proteins. The relative ease/low cost of synthesis of PAMAM dendrimers (especially relative to similarly-sized biological molecules such as proteins and antibodies), along with their biocompatibility, structural control, and functionalizability, have made PAMAMs viable candidates for application in drug development, biochemistry, and nanotechnology.
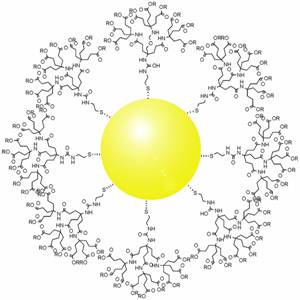
Gold nanoparticles in chemotherapy and radiotherapy is the use of colloidal gold in therapeutic treatments, often for cancer or arthritis. Gold nanoparticle technology shows promise in the advancement of cancer treatments. Some of the properties that gold nanoparticles possess, such as small size, non-toxicity and non-immunogenicity make these molecules useful candidates for targeted drug delivery systems. With tumor-targeting delivery vectors becoming smaller, the ability to by-pass the natural barriers and obstacles of the body becomes more probable. To increase specificity and likelihood of drug delivery, tumor specific ligands may be grafted onto the particles along with the chemotherapeutic drug molecules, to allow these molecules to circulate throughout the tumor without being redistributed into the body.
Hamid Ghandehari is an Iranian-American drug delivery research scientist, and a professor in the Departments of Pharmaceutics and Pharmaceutical Chemistry and Biomedical Engineering at the University of Utah. His research is focused in recombinant polymers for drug and gene delivery, nanotoxicology of dendritic and inorganic constructs, water-soluble polymers for targeted delivery and poly(amidoamine) dendrimers for oral delivery.
A protein corona is a dynamic coating of biomolecules, usually proteins, around the surface of a nanoparticle that forms spontaneously in colloidal nanomaterials upon exposure to biological mediums. Protein coronas can form in many different patterns depending on their size, shape, composition, charge, and surface functional groups, and have properties that vary in different environmental factors like temperature, pH, shearing stress, immersed media composition, and exposing time. These coatings are also changeable according to the conditions of the biochemical and physiochemical surface interactions. Types of protein coronas are known to be divided into two categories: “hard” and “soft”. “Hard” coronas have higher-affinity proteins that are irreversibly bonded to the nanoparticle surface, while “soft” coronas have lower-affinity proteins on the nanoparticle surface that are reversibly bound. These reversibly-bound proteins allow for the biomolecules in “soft” protein coronas to be exchanged or detached over time for various applications. This process is governed by the intermolecular protein-nanoparticle and protein-protein interactions that exist within a solution. In "soft" protein coronas, it is common to observe an exchange of proteins at the surface; larger proteins with lower affinities will often aggregate to the surface of the nanoparticle first, and over time, smaller proteins with higher affinities will replace them, "hardening" the corona, known as the Vroman effect.
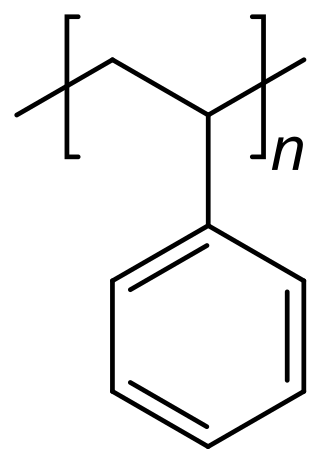
Polystyrene is a synthetic hydrocarbon polymer that is widely adaptive and can be used for a variety of purposes in drug delivery. These methods include polystyrene microspheres, nanoparticles, and solid foams. In the biomedical engineering field, these methods assist researchers in drug delivery, diagnostics, and imaging strategies.
pH-responsive tumor-targeted drug delivery is a specialized form of targeted drug delivery that utilizes nanoparticles to deliver therapeutic drugs directly to cancerous tumor tissue while minimizing its interaction with healthy tissue. Scientists have used drug delivery as a way to modify the pharmacokinetics and targeted action of a drug by combining it with various excipients, drug carriers, and medical devices. These drug delivery systems have been created to react to the pH environment of diseased or cancerous tissues, triggering structural and chemical changes within the drug delivery system. This form of targeted drug delivery is to localize drug delivery, prolongs the drug's effect, and protect the drug from being broken down or eliminated by the body before it reaches the tumor.
Intranasal drug delivery occurs when particles are inhaled into the nasal cavity and transported directly into the nervous system. Though pharmaceuticals can be injected into the nose, some concerns include injuries, infection, and safe disposal. Studies demonstrate improved patient compliance with inhalation. Treating brain diseases has been a challenge due to the blood brain barrier. Previous studies evaluated the efficacy of delivery therapeutics through intranasal route for brain diseases and mental health conditions. Intranasal administration is a potential route associated with high drug transfer from nose to brain and drug bioavailability.

A ligand-targeted liposome (LTL) is a nanocarrier with specific ligands attached to its surface to enhance localization for targeted drug delivery. The targeting ability of LTLs enhances cellular localization and uptake of these liposomes for therapeutic or diagnostic purposes. LTLs have the potential to enhance drug delivery by decreasing peripheral systemic toxicity, increasing in vivo drug stability, enhancing cellular uptake, and increasing efficiency for chemotherapeutics and other applications. Liposomes are beneficial in therapeutic manufacturing because of low batch-to-batch variability, easy synthesis, favorable scalability, and strong biocompatibility. Ligand-targeting technology enhances liposomes by adding targeting properties for directed drug delivery.











