Neutrophil cytosol factor 4 is a protein that in humans is encoded by the NCF4 gene. [5] [6]
Neutrophil cytosol factor 4 is a protein that in humans is encoded by the NCF4 gene. [5] [6]
The protein encoded by this gene is a cytosolic regulatory component of the superoxide-producing phagocyte NADPH-oxidase, a multicomponent enzyme system important for host defense. This protein is preferentially expressed in cells of myeloid lineage. It interacts primarily with neutrophil cytosolic factor 2 (NCF2/p67-phox) to form a complex with neutrophil cytosolic factor 1 (NCF1/p47-phox), which further interacts with the small G protein RAC1 and translocates to the membrane upon cell stimulation. This complex then activates flavocytochrome b, the membrane-integrated catalytic core of the enzyme system. The PX domain of this protein can bind phospholipid products of the PI(3) kinase, which suggests its role in PI(3) kinase-mediated signaling events. The phosphorylation of this protein was found to negatively regulate the enzyme activity. Alternatively spliced transcript variants encoding distinct isoforms have been observed.
GWAS studies showed that Crohn's disease patient with certain SNPs in NCF4 are more susceptible to get Crohn's disease. [7] Crohn's patient with rs4821544 variants showed a decreased reactive oxygen species after stimulation with GM-CSF which is a proinflammtory cytokine. [8]
Neutrophil cytosolic factor 4 has been shown to interact with Ku70, [9] Neutrophil cytosolic factor 1 [10] [11] [12] and Moesin. [13]

Chronic granulomatous disease (CGD), also known as Bridges–Good syndrome, chronic granulomatous disorder, and Quie syndrome, is a diverse group of hereditary diseases in which certain cells of the immune system have difficulty forming the reactive oxygen compounds used to kill certain ingested pathogens. This leads to the formation of granulomas in many organs. CGD affects about 1 in 200,000 people in the United States, with about 20 new cases diagnosed each year.
Respiratory burst is the rapid release of the reactive oxygen species (ROS), superoxide anion and hydrogen peroxide, from different cell types.
NADPH oxidase is a membrane-bound enzyme complex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white blood cells to engulf microorganisms. Human isoforms of the catalytic component of the complex include NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2.

NADPH oxidase 2 (Nox2), also known as cytochrome b(558) subunit beta or Cytochrome b-245 heavy chain, is a protein that in humans is encoded by the NOX2 gene. The protein is a superoxide generating enzyme which forms reactive oxygen species (ROS).
Anthony (Tony) Segal MD PhD FRS FMedSci is a British physician/scientist.

An alveolar macrophage, pulmonary macrophage, is a type of macrophage, a professional phagocyte, found in the airways and at the level of the alveoli in the lungs, but separated from their walls.
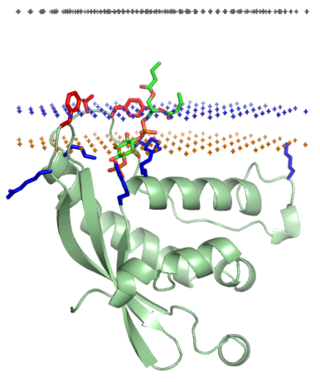
The PX domain is a phosphoinositide-binding structural domain involved in targeting of proteins to cell membranes.

Putative neutrophil cytosol factor 1C is a protein that in humans is encoded by the NCF1C gene. It relates to a type of white blood cell called a neutrophil. The Neutrophil Cytosolic Factor 1C (NCF1C) gene is responsible for encoding the 47 kDA cytosolic subunit of NADPH oxidase. The NCF1C gene is located near two pseudogenes and when the NCF1C gene recombines with them, the NCF1C gene will inactivate and can lead to chronic granulomatous disease.

Neutrophil cytosol factor 2 is a protein that in humans is encoded by the NCF2 gene.

Neutrophil cytosol factor 1, also known as p47phox, is a protein that in humans is encoded by the NCF1 gene.

NADPH oxidase 1 is an enzyme that in humans is encoded by the NOX1 gene.

Cytochrome b-245 light chain is a protein that in humans is encoded by the CYBA gene involved in superoxide production and phagocytosis.

RHO protein GDP dissociation inhibitor of Rho proteins, regulates GDP/GTP exchange.
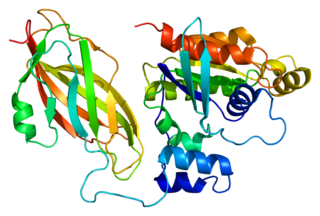
Rac2 is a small signaling G protein, and is a member of the Rac subfamily of the family Rho family of GTPases. It is encoded by the gene RAC2.

Dual oxidase 2, also known as DUOX2 or ThOX2, is an enzyme that in humans is encoded by the DUOX2 gene. Dual oxidase is an enzyme that was first identified in the mammalian thyroid gland. In humans, two isoforms are found; hDUOX1 and hDUOX2. The protein location is not exclusive to thyroid tissue; hDUOX1 is prominent in airway epithelial cells and hDUOX2 in the salivary glands and gastrointestinal tract.

NADPH oxidase, EF-hand calcium binding domain 5, also known as NOX5, is a protein which in humans is encoded by the NOX5 gene.

NADPH oxidase organizer 1 is an enzyme that in humans is encoded by the NOXO1 gene.

NADPH oxidase 3 is an enzyme that in humans is encoded by the NOX3 gene.
p22phox Protein, also known as the human neutrophil cytochrome b light chain (CYBA), is an essential component of the membrane-associated enzyme phagocyte NADPH-oxidase This enzyme uses NADH or NADPH as the electron donor for the one electron reduction of oxygen to produce superoxide anion, a reactive oxygen species (ROS), and a functionally important step for the antimicrobial activity of phagocytic cells. p22phox is also expressed in many other human cells such as endothelial and vascular smooth muscle cells, including those within the coronary arteries. Specific polymorphisms of the CYBA gene have been identified that are associated with a decreased risk of coronary artery disease (CAD).
Edgar Pick is an Israeli immunologist who is Professor Emeritus of Immunology in the Department of Clinical Microbiology and Immunology at the Sackler Faculty of Medicine at Tel Aviv University, Israel.