Related Research Articles

Alkaloids are a class of naturally occurring organic compounds that mostly contain basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.

Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.

Nuphar is a genus of aquatic plants in the family Nymphaeaceae, with a temperate to subarctic Northern Hemisphere distribution. Common names include water-lily, pond-lily, alligator-bonnet or bonnet lily, and spatterdock.

Nymphaea lotus, the white Egyptian lotus, tiger lotus, white lotus or Egyptian white water-lily, is a flowering plant of the family Nymphaeaceae.
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. It contains a five-membered ring consisting of four carbon atoms and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, colorless liquid with an intensely unpleasant odor. It is also known as thiophane, thiolane, or THT.

Voacangine is an alkaloid found predominantly in the rootbark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has also been demonstrated in animals to have similar anti-addictive properties to ibogaine itself.

Nuphar lutea, the yellow water-lily, or brandy-bottle, is an aquatic plant of the family Nymphaeaceae, native to temperate regions of Europe, northwest Africa, and western Asia.

Nuphar advena is a species of Nuphar native throughout the eastern United States and at some parts of Canada, such as Nova Scotia. It is similar to the Eurasian species N. lutea, and is treated as a subspecies of it by some botanists, though differing significantly in genetics.

Akuammine (vincamajoridine) is an indole alkaloid. It is the most abundant alkaloid found in the seeds from the tree Picralima nitida, commonly known as akuamma, comprising 0.56% of the dried powder. It has also been isolated from Vinca major. Akuammine is structurally related to both yohimbine and mitragynine, both of which are alkaloid plant products with pharmacological properties.
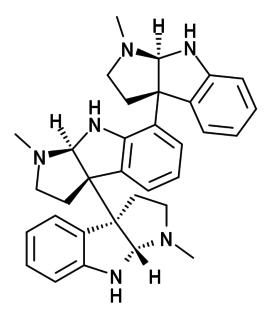
Hodgkinsine is an alkaloid found in plants of the genus Psychotria, particularly Psychotria colorata, although it is also found in Psychotria lyciiflora and probably other species in this family,

Osman Achmatowicz was a Polish professor of chemistry of Lipka Tatar descent. His son, Osman Achmatowicz Jr., is credited with the Achmatowicz reaction in 1971.

Pericine is one of a number of indole alkaloids found in the tree Picralima nitida, commonly known as akuamma. As with some other alkaloids from this plant such as akuammine, pericine has been shown to bind to mu opioid receptors in vitro, and has an IC50 of 0.6 μmol, within the range of a weak analgesic. It may also have convulsant effects.
The molecular formula C15H25NO2 may refer to:

Ibogamine is an alkaloid found in Tabernanthe iboga.

Nuphar pumila, the least water-lily or small yellow pond-lily, is an aquatic perennial plant in the Nymphaeaceae family. It is also known as dwarf water lily because it is the dwarf species of Nuphar lutea; while Nuphar pumila has a star-shaped, or lobed form of the stigma disc and glabrous leaf undersides, Nuphar lutea has a round stigma disc and the undersides of its leaves are occasionally fine-haired on the midribs. Its flowers bloom from July to August and are typically pollinated by flies.

Nuphar polysepala is a species of Nuphar native to western North America. The name Nuphar is Greek for "water-lily" and polysepala means many sepals. It is commonly found in shallow muddy ponds from northern Alaska and Yukon southward to central California and northern New Mexico, and can be recognized easily by its large floating leaves and bright yellow blossoms.

Nuphar japonica, known as East Asian yellow water-lily, is an aquatic plant species in the genus Nuphar found in Japan and the Korean Peninsula. It is endangered in Russia. The species was not accepted by The Plant List as of November 2013, which regarded it as an "unresolved name".
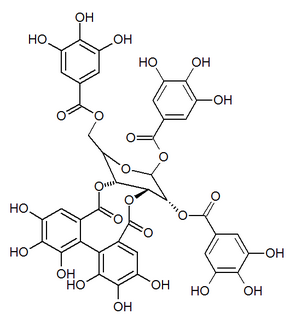
Nupharin A is an ellagitannin found in Nuphar japonica. It is a molecule with three gallic acid units and one hexahydroxydiphenic acid unit attached to a glucose residue. It is an isomer of punicafolin and tellimagrandin II.

Nupharamine is an alkaloid found in Nuphar japonica and in castoreum.

Neothiobinupharidine is a bio-active alkaloid isolated from Nuphar pumila.
References
- ↑ Chapter 10 Nuphar Alkaloids. J.T. Wróbel, The Alkaloids: Chemistry and Physiology, 1967, Volume 9, Pages 441–465, doi : 10.1016/S1876-0813(08)60206-7
| This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |