
An oil bath is a type of heated bath used in a laboratory, most commonly used to heat up chemical reactions. It's essentially a container of oil that is heated by a hot plate or (in rare cases) a Bunsen burner.

An oil bath is a type of heated bath used in a laboratory, most commonly used to heat up chemical reactions. It's essentially a container of oil that is heated by a hot plate or (in rare cases) a Bunsen burner.
These baths are commonly used to heat reaction mixtures more evenly than would be possible with a hot plate alone, as the entire outside of the reaction flask is heated. Generally, silicone oil is used in modern oil baths, although mineral oil, cottonseed oil and even phosphoric acid have been used in the past. [1]
Overheating the oil bath can result in a fire hazard, especially if mineral oil is being used. Generally, the maximum safe operating temperature of a mineral oil bath is approximately 160 °C (320 °F), the oil's flash point. Mineral oil can't be used above 310 °C (590 °F) due to the compound's boiling point. If higher temperatures are needed, a silicone oil or a sand bath may be used instead. [2] [ better source needed ] Silicone oil baths are effective in the 25 °C (77 °F) - 230 °C (446 °F) range. Sand baths are effective from 25 °C (77 °F) to above 500 °C (932 °F). [3]

Another use of an oil bath is to filter particulates out of air, by leading the air stream through an unheated oil bath. This type of air filter was used in car and tractor engines, but has been replaced by modern paper air filters; some small engines continue to use this system. In some cases oil baths are used to heat bearings so they expand before installing them on shafts of aircraft engines and tractors.
A lubricant is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, transporting foreign particles, or heating or cooling the surfaces. The property of reducing friction is known as lubricity.

A magnetic stirrer or magnetic mixer is a laboratory device that employs a rotating magnetic field to cause a stir bar immersed in a liquid to spin very quickly, thus stirring it. The rotating field may be created either by a rotating magnet or a set of stationary electromagnets, placed beneath the vessel with the liquid. It is used in chemistry and biology where other forms of stirring, such as motorized stirrers and stirring rods, may not be viable for use.

Heat treating is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve the desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching. Although the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.

A hydraulic fluid or hydraulic liquid is the medium by which power is transferred in hydraulic machinery. Common hydraulic fluids are based on mineral oil or water. Examples of equipment that might use hydraulic fluids are excavators and backhoes, hydraulic brakes, power steering systems, automatic transmissions, garbage trucks, aircraft flight control systems, lifts, and industrial machinery.

A diving air compressor is a gas compressor that can provide breathing air directly to a surface-supplied diver, or fill diving cylinders with high-pressure air pure enough to be used as a breathing gas. A low pressure diving air compressor usually has a delivery pressure of up to 30 bar, which is regulated to suit the depth of the dive. A high pressure diving compressor has a delivery pressure which is usually over 150 bar, and is commonly between 200 and 300 bar. The pressure is limited by an overpressure valve which may be adjustable.

A solar thermal collector collects heat by absorbing sunlight. The term "solar collector" commonly refers to a device for solar hot water heating, but may refer to large power generating installations such as solar parabolic troughs and solar towers or non water heating devices such as solar air heaters.

A hot plate is a portable self-contained tabletop small appliance cooktop that features one or more electric heating elements or gas burners. A hot plate can be used as a stand-alone appliance, but is often used as a substitute for one of the burners from an oven range or a kitchen stove. Hot plates are often used for food preparation, generally in locations where a full kitchen stove would not be convenient or practical. They can also be used as a heat source in laboratories. A hot plate can have a flat surface or round surface. Hot plates can be used for traveling or in areas without electricity.

The thermal cycler is a laboratory apparatus most commonly used to amplify segments of DNA via the polymerase chain reaction (PCR). Thermal cyclers may also be used in laboratories to facilitate other temperature-sensitive reactions, including restriction enzyme digestion or rapid diagnostics. The device has a thermal block with holes where tubes holding the reaction mixtures can be inserted. The cycler then raises and lowers the temperature of the block in discrete, pre-programmed steps.
A coolant is a substance, typically liquid or gas, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosion of the cooling system. Some applications also require the coolant to be an electrical insulator.
Silicone grease, sometimes called dielectric grease, is a waterproof grease made by combining a silicone oil with a thickener. Most commonly, the silicone oil is polydimethylsiloxane (PDMS) and the thickener is amorphous fumed silica. Using this formulation, silicone grease is a translucent white viscous paste, with exact properties dependent on the type and proportion of the components. More specialized silicone greases are made from fluorinated silicones or, for low-temperature applications, PDMS containing some phenyl substituents in place of methyl groups. Other thickeners may be used, including stearates and powdered polytetrafluorethylene (PTFE). Greases formulated from silicone oils with silica thickener are sometimes referred to as silicone paste to distinguish them from silicone grease made with silicone oil and a soap thickener.
Carburetor, carburettor, carburator, carburettor heat is a system used in automobile and piston-powered light aircraft engines to prevent or clear carburetor icing. It consists of a moveable flap which draws hot air into the engine intake. The air is drawn from the heat stove, a metal plate around the exhaust manifold.
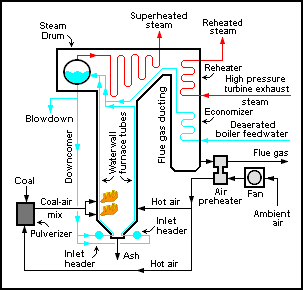
An air preheater is any device designed to heat air before another process (for example, combustion in a boiler With the primary objective of increasing the thermal efficiency of the process. They may be used alone or to replace a recuperative heat system or to replace a steam coil.

A sand bath is a common piece of laboratory equipment made from a container filled with heated sand. It is used to provide even heating for another container, most often during a chemical reaction.
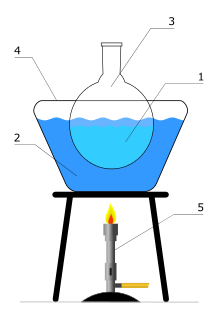
A heated bath is used in the laboratory to allow a chemical reaction to occur at an elevated temperature.

Furo, or the more common and polite form ofuro, is a Japanese bath and/or bathroom. Specifically it is a type of bath which originated as a short, steep-sided wooden bathtub. Baths of this type are found all over Japan in houses, apartments and traditional Japanese inns (ryokan) but are now usually made out of a plastic or stainless steel.

A thermal oxidizer is a process unit for air pollution control in many chemical plants that decomposes hazardous gases at a high temperature and releases them into the atmosphere.

A cooling bath or ice bath, in laboratory chemistry practice, is a liquid mixture which is used to maintain low temperatures, typically between 13 °C and −196 °C. These low temperatures are used to collect liquids after distillation, to remove solvents using a rotary evaporator, or to perform a chemical reaction below room temperature.
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time.

A water bath is laboratory equipment made from a container filled with heated water. It is used to incubate samples in water at a constant temperature over a long period of time. Most water baths have a digital or an analogue interface to allow users to set a desired temperature, but some water baths have their temperature controlled by a current passing through a reader. Utilisations include warming of reagents, melting of substrates or incubation of cell cultures. It is also used to enable certain chemical reactions to occur at high temperature. Water baths are preferred heat sources for heating flammable chemicals, as their lack of open flame prevents ignition. Different types of water baths are used depending on application. For all water baths, it can be used up to 99.9 °C. When temperature is above 100 °C, alternative methods such as oil bath, silicone bath or sand bath may be used.
Compressed air dryers are special types of filter systems that are specifically designed to remove the water that is inherent in compressed air. The process of compressing air raises its temperature and concentrates atmospheric contaminants, primarily water vapor. Consequently, the compressed air is generally at an elevated temperature and 100% relative humidity. As the compressed air cools, water vapor condenses into the tank(s), pipes, hoses and tools that are downstream from the compressor. Water vapor is removed from compressed air to prevent condensation from occurring and to prevent moisture from interfering in sensitive industrial processes.