Halogenation is a chemical reaction that involves the addition of one or more halogens to a compound or material. The pathway and stoichiometry of halogenation depends on the structural features and functional groups of the organic substrate, as well as on the specific halogen. Inorganic compounds such as metals also undergo halogenation.

An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula HSO3F. It is one of the strongest acids commercially available. The formula HSO3F emphasizes its relationship to sulfuric acid, H2SO4; HSO3F is a tetrahedral molecule. It is a colourless liquid, although commercial samples are often yellow.

Cobalt(III) fluoride is the inorganic compound with the formula CoF
3. Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.

Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound. Silver usually exists in its +1 oxidation state. It is used as a fluorinating agent.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with light or water vapor but is otherwise stable in storage. Xenon difluoride is a dense, white crystalline solid.
Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swart's reagent, is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid. As well as some industrial applications, it is used as a reagent in inorganic and organofluorine chemistry.
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

Hypofluorous acid, chemical formula HOF, is the only known oxoacid of fluorine and the only known oxoacid which the main atom gains electrons from oxygen to create a negative oxidation state. The oxidation state of the oxygen in hypofluorites is 0. It is also the only hypohalous acid that can be isolated as a solid. HOF is an intermediate in the oxidation of water by fluorine, which produces hydrogen fluoride, oxygen difluoride, hydrogen peroxide, ozone and oxygen. HOF is explosive at room temperature, forming HF and O2:

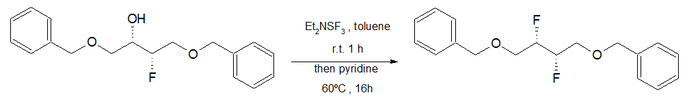
Diethylaminosulfur trifluoride (DAST) is the organosulfur compound with the formula Et2NSF3. This liquid is a fluorinating reagent used for the synthesis of organofluorine compounds. The compound is colourless; older samples assume an orange colour.

Cyanuric fluoride or 2,4,6-trifluoro-1,3,5-triazine is a chemical compound with the formula (CNF)3. It is a colourless, pungent liquid. It has been used as a precursor for fibre-reactive dyes, as a specific reagent for tyrosine residues in enzymes, and as a fluorinating agent.
1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) or Selectfluor, a trademark of Air Products and Chemicals, is a reagent in chemistry that is used as a fluorine donor. This compound is a derivative of the nucleophillic base DABCO. This colourless salt was first described in 1992 and has since been commercialized for use in organofluorine chemistry for electrophilic fluorination.
The Fowler process is an industry and laboratory route to fluorocarbons, by fluorinating hydrocarbons or their partially fluorinated derivatives in the vapor phase over cobalt(III) fluoride.

Plutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium. This pure plutonium is needed to avoid premature ignition of low-mass nuclear weapon designs by neutrons produced by spontaneous fission of plutonium-240.
Fluorination by sulfur tetrafluoride produces organofluorine compounds from oxidized organic compounds, including alcohols, carbonyl compounds, alkyl halides, and others.
Fluorination with aminosulfuranes is a chemical reaction that transforms oxidized organic compounds into organofluorine compounds. Aminosulfuranes selectively exchange hydroxyl groups for fluorine, but are also capable of converting carbonyl groups, halides, silyl ethers, and other functionality into organofluorides.
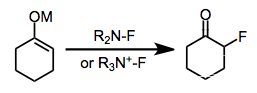
Electrophilic fluorination is the combination of a carbon-centered nucleophile with an electrophilic source of fluorine to afford organofluorine compounds. Although elemental fluorine and reagents incorporating an oxygen-fluorine bond can be used for this purpose, they have largely been replaced by reagents containing a nitrogen-fluorine bond.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Fluoroalcohols are organofluorine compounds consisting of an alcohol functional group with at least one C-F bond. These compounds often have distinctive solvent properties.