
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. Rarer still, they may contain elements such as phosphorus, chlorine, and bromine.

Secondary metabolites, also called specialised metabolites, secondary products, or natural products, are organic compounds produced by any lifeform, e.g. bacteria, fungi, animals, or plants, which are not directly involved in the normal growth, development, or reproduction of the organism. Instead, they generally mediate ecological interactions, which may produce a selective advantage for the organism by increasing its survivability or fecundity. Specific secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavourings, pigments, and recreational drugs.

Porphyria cutanea tarda is the most common subtype of porphyria. The disease is named because it is a porphyria that often presents with skin manifestations later in life. The disorder results from low levels of the enzyme responsible for the fifth step in heme production. Heme is a vital molecule for all of the body's organs. It is a component of hemoglobin, the molecule that carries oxygen in the blood.
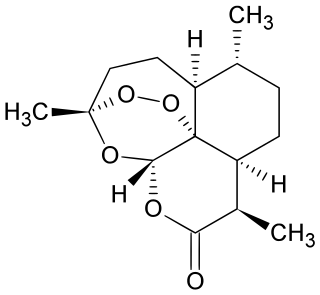
Artemisinin and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery. Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium. Artemisinin is extracted from the plant Artemisia annua an herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.

Lactucopicrin (Intybin) is a bitter substance that has a sedative and analgesic effect, acting on the central nervous system. It is a sesquiterpene lactone, and is a component of lactucarium, derived from the plant Lactuca virosa, as well as being found in some related plants such as Cichorium intybus. It is also found in dandelion coffee.

Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.
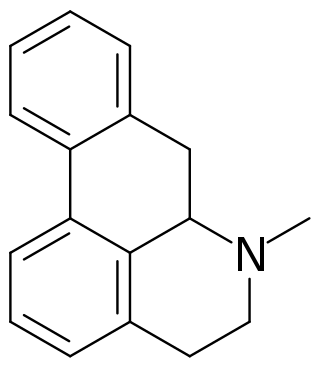
Aporphine is an alkaloid with the chemical formula C17H17N. It is the core chemical substructure of the aporphine alkaloids, a subclass of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.

Cycloguanil is a dihydrofolate reductase inhibitor, and is a metabolite of the antimalarial drug proguanil; its formation in vivo has been thought to be primarily responsible for the antimalarial activity of proguanil. However, more recent work has indicated that, while proguanil is synergistic with the drug atovaquone, cycloguanil is in fact antagonistic to the effects of atovaquone, suggesting that, unlike cycloguanil, proguanil may have an alternative mechanism of antimalarial action besides dihydrofolate reductase inhibition.

Akuammine (vincamajoridine) is an indole alkaloid. It is the most abundant alkaloid found in the seeds from the tree Picralima nitida, commonly known as akuamma, comprising 0.56% of the dried powder. It has also been isolated from Vinca major. Akuammine is structurally related to yohimbine, mitragynine and more distantly Voacangine, all of which are alkaloid plant products with pharmacological properties.

Pukateine is an alkaloid found in the bark of the New Zealand tree Laurelia novae-zelandiae ("Pukatea"), as well as some South American plants. An extract from pukatea is used in traditional Māori herbal medicine as an analgesic.

Conessine is a steroidal alkaloid found in a number of plant species from the family Apocynaceae, including Holarrhena floribunda, Holarrhena antidysenterica and Funtumia elastica. It acts as a histamine antagonist, selective for the H3 subtype (with an affinity of pKi = 8.27; Ki = ~5 nM). It was also found to have long CNS clearance times, high blood–brain barrier penetration and high affinity for the adrenergic receptors.
Keller's reagent can refer to either of two different mixtures of acids.

Coronaridine, also known as 18-carbomethoxyibogamine, is an alkaloid found in Tabernanthe iboga and related species, including Tabernaemontana divaricata for which it was named.
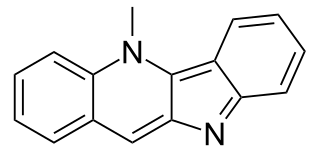
Cryptolepine is an alkaloid with antimalarial and cytotoxic properties, in vitro and in mice. It is able to intercalate into DNA at the cytosine-cytosine sites. Because of its toxicity, Cryptolepine is not considered appropriate for use as an anti-malarial drug in humans.

Picralima is a plant genus in the family Apocynaceae, first described as a genus in 1896. It contains only one known species, Picralima nitida, native to tropical Africa.

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

(S)-Cheilanthifoline is a benzylisoquinoline alkaloid (BIA) which has been isolated from Corydalis dubia and Argemone mexicana. (S)-Cheilanthifoline is metabolically derived from (S)-reticuline, a pivotal intermediate in the biosynthesis of numerous BIAs. (S)-Cheilanthifoline is the immediate precursor of the BIA (S)-stylopine ((S)-stylopine synthase/CYP719A20), which is the precursor for the alkaloids protopine and sanguinarine.

Quassinoids are degraded triterpene lactones of the Simaroubaceae plant family grouped into C-18, C-19, C-20, C-22 and C-25 types. The prototypical member of the group, quassin, was first described in the 19th century from plants of the genus Quassia from which it gets its name. It was isolated in 1937, and its structure elucidated in 1961.

Mitragynine pseudoindoxyl is a rearrangement product of 7-hydroxymitragynine an active metabolite of mitragynine.

Tabernaemontanine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.



















