Related Research Articles

In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sucrose and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.

In organic chemistry, a ketone is a functional group with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula CH3. In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond, it can be found on its own in any of three forms: methanide anion, methylium cation or methyl radical. The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
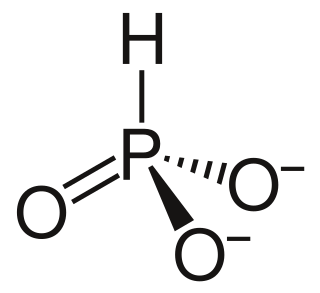
A phosphite anion or phosphite in inorganic chemistry usually refers to [HPO3]2− but includes [H2PO3]− ([HPO2(OH)]−). These anions are the conjugate bases of phosphorous acid (H3PO3). The corresponding salts, e.g. sodium phosphite (Na2HPO3) are reducing in character.
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula −C4H9, derived from either of the two isomers (n-butane and isobutane) of butane.

Catechol, also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula C6H4(OH)2. It is the ortho isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances.

An organic acid anhydride is an acid anhydride that is also an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride. Thus, (CH3CO)2O is called acetic anhydride.Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride").
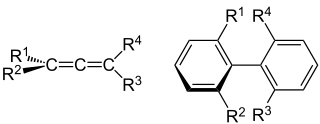
In chemistry, axial chirality is a special case of chirality in which a molecule contains two pairs of chemical groups in a non-planar arrangement about an axis of chirality so that the molecule is not superposable on its mirror image. The axis of chirality is usually determined by a chemical bond that is constrained against free rotation either by steric hindrance of the groups, as in substituted biaryl compounds such as BINAP, or by torsional stiffness of the bonds, as in the C=C double bonds in allenes such as glutinic acid. Axial chirality is most commonly observed in substituted biaryl compounds wherein the rotation about the aryl–aryl bond is restricted so it results in chiral atropisomers, as in various ortho-substituted biphenyls, and in binaphthyls such as BINAP.
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.

Orthocarbonic acid, carbon hydroxide or methanetetrol is the name given to a hypothetical compound with the chemical formula H4CO4 or C(OH)4. Its molecular structure consists of a single carbon atom bonded to four hydroxy groups. It would be therefore a fourfold alcohol. In theory it could lose four protons to give the hypothetical oxocarbon anion orthocarbonateCO4−4, and is therefore considered an oxoacid of carbon.
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid.

Phthalaldehyde (sometimes also o-phthalaldehyde or ortho-phthalaldehyde, OPA) is the chemical compound with the formula C6H4(CHO)2. It is one of three isomers of benzene dicarbaldehyde, related to phthalic acid. This pale yellow solid is a building block in the synthesis of heterocyclic compounds and a reagent in the analysis of amino acids. OPA dissolves in water solution at pH < 11.5. Its solutions degrade upon UV illumination and exposure to air.
In chemical nomenclature, a descriptor is a notational prefix placed before the systematic substance name, which describes the configuration or the stereochemistry of the molecule. Some listed descriptors are only of historical interest and should not be used in publications anymore as they do not correspond with the modern recommendations of the IUPAC. Stereodescriptors are often used in combination with locants to clearly identify a chemical structure unambiguously.
References
- ↑ IUPAC , Compendium of Chemical Terminology , 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) " ortho acids ". doi : 10.1351/goldbook.O04331