Related Research Articles
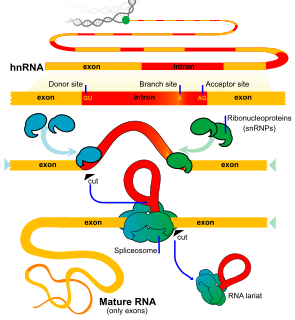
An exon is any part of a gene that will form a part of the final mature RNA produced by that gene after introns have been removed by RNA splicing. The term exon refers to both the DNA sequence within a gene and to the corresponding sequence in RNA transcripts. In RNA splicing, introns are removed and exons are covalently joined to one another as part of generating the mature RNA. Just as the entire set of genes for a species constitutes the genome, the entire set of exons constitutes the exome.
An intron is any nucleotide sequence within a gene that is removed by RNA splicing during maturation of the final RNA product. In other words, introns are non-coding regions of an RNA transcript, or the DNA encoding it, that are eliminated by splicing before translation. The word intron is derived from the term intragenic region, i.e. a region inside a gene. The term intron refers to both the DNA sequence within a gene and the corresponding sequence in RNA transcripts. Sequences that are joined in the final mature RNA after RNA splicing are exons.

Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid (DNA) are nucleic acids. Along with lipids, proteins, and carbohydrates, nucleic acids constitute one of the four major macromolecules essential for all known forms of life. Like DNA, RNA is assembled as a chain of nucleotides, but unlike DNA, RNA is found in nature as a single strand folded onto itself, rather than a paired double strand. Cellular organisms use messenger RNA (mRNA) to convey genetic information that directs synthesis of specific proteins. Many viruses encode their genetic information using an RNA genome.

RNA splicing is a process in molecular biology where a newly-made precursor messenger RNA (pre-mRNA) transcript is transformed into a mature messenger RNA (mRNA). It works by removing introns and so joining together exons. For nuclear-encoded genes, splicing occurs in the nucleus either during or immediately after transcription. For those eukaryotic genes that contain introns, splicing is usually needed to create an mRNA molecule that can be translated into protein. For many eukaryotic introns, splicing occurs in a series of reactions which are catalyzed by the spliceosome, a complex of small nuclear ribonucleoproteins (snRNPs). There exist self-splicing introns, that is, ribozymes that can catalyze their own excision from their parent RNA molecule.

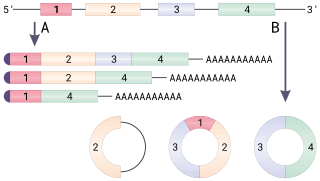
Alternative splicing, or alternative RNA splicing, or differential splicing, is an alternative splicing process during gene expression that allows a single gene to code for multiple proteins. In this process, particular exons of a gene may be included within or excluded from the final, processed messenger RNA (mRNA) produced from that gene. This means the exons are joined in different combinations, leading to different (alternative) mRNA strands. Consequently, the proteins translated from alternatively spliced mRNAs will contain differences in their amino acid sequence and, often, in their biological functions. Notably, alternative splicing allows the human genome to direct the synthesis of many more proteins than would be expected from its 20,000 protein-coding genes.
Trans-splicing is a special form of RNA processing where exons from two different primary RNA transcripts are joined end to end and ligated. It is usually found in eukaryotes and mediated by the spliceosome, although some bacteria and archaea also have "half-genes" for tRNAs.
Post-transcriptional modification or co-transcriptional modification is a set of biological processes common to most eukaryotic cells by which an RNA primary transcript is chemically altered following transcription from a gene to produce a mature, functional RNA molecule that can then leave the nucleus and perform any of a variety of different functions in the cell. There are many types of post-transcriptional modifications achieved through a diverse class of molecular mechanisms.
Exon shuffling is a molecular mechanism for the formation of new genes. It is a process through which two or more exons from different genes can be brought together ectopically, or the same exon can be duplicated, to create a new exon-intron structure. There are different mechanisms through which exon shuffling occurs: transposon mediated exon shuffling, crossover during sexual recombination of parental genomes and illegitimate recombination.

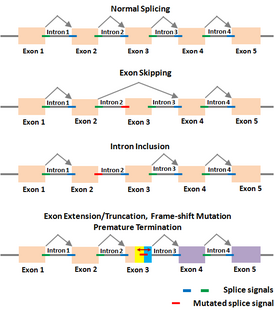
A splice site mutation is a genetic mutation that inserts, deletes or changes a number of nucleotides in the specific site at which splicing takes place during the processing of precursor messenger RNA into mature messenger RNA. Splice site consensus sequences that drive exon recognition are located at the very termini of introns. The deletion of the splicing site results in one or more introns remaining in mature mRNA and may lead to the production of abnormal proteins. When a splice site mutation occurs, the mRNA transcript possesses information from these introns that normally should not be included. Introns are supposed to be removed, while the exons are expressed.
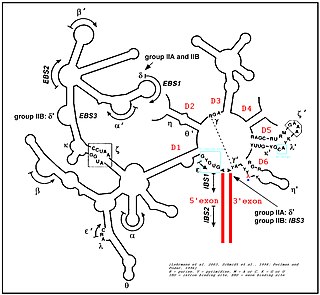
Group II introns are a large class of self-catalytic ribozymes and mobile genetic elements found within the genes of all three domains of life. Ribozyme activity can occur under high-salt conditions in vitro. However, assistance from proteins is required for in vivo splicing. In contrast to group I introns, intron excision occurs in the absence of GTP and involves the formation of a lariat, with an A-residue branchpoint strongly resembling that found in lariats formed during splicing of nuclear pre-mRNA. It is hypothesized that pre-mRNA splicing may have evolved from group II introns, due to the similar catalytic mechanism as well as the structural similarity of the Group II Domain V substructure to the U6/U2 extended snRNA. Finally, their ability to site-specifically mobilize to new DNA sites has been exploited as a tool for biotechnology.
An exonic splicing silencer (ESS) is a short region of an exon and is a cis-regulatory element. A set of 103 hexanucleotides known as FAS-hex3 has been shown to be abundant in ESS regions. ESSs inhibit or silence splicing of the pre-mRNA and contribute to constitutive and alternate splicing. To elicit the silencing affect, ESSs recruit proteins that will negatively affect the core splicing machinery.

U2 spliceosomal snRNAs are a species of small nuclear RNA (snRNA) molecules found in the major spliceosomal (Sm) machinery of virtually all eukaryotic organisms. In vivo, U2 snRNA along with its associated polypeptides assemble to produce the U2 small nuclear ribonucleoprotein (snRNP), an essential component of the major spliceosomal complex. The major spliceosomal-splicing pathway is occasionally referred to as U2 dependent, based on a class of Sm intron—found in mRNA primary transcripts—that are recognized exclusively by the U2 snRNP during early stages of spliceosomal assembly. In addition to U2 dependent intron recognition, U2 snRNA has been theorized to serve a catalytic role in the chemistry of pre-RNA splicing as well. Similar to ribosomal RNAs (rRNAs), Sm snRNAs must mediate both RNA:RNA and RNA:protein contacts and hence have evolved specialized, highly conserved, primary and secondary structural elements to facilitate these types of interactions.

In molecular genetics, an untranslated region refers to either of two sections, one on each side of a coding sequence on a strand of mRNA. If it is found on the 5' side, it is called the 5' UTR, or if it is found on the 3' side, it is called the 3' UTR. mRNA is RNA that carries information from DNA to the ribosome, the site of protein synthesis (translation) within a cell. The mRNA is initially transcribed from the corresponding DNA sequence and then translated into protein. However, several regions of the mRNA are usually not translated into protein, including the 5' and 3' UTRs.
Trans-Spliced Exon Coupled RNA End Determination (TEC-RED) is a transcriptomic technique that, like SAGE, allows for the digital detection of messenger RNA sequences. Unlike SAGE, detection and purification of transcripts from the 5’ end of the messenger RNA require the presence of a trans-spliced leader sequence.
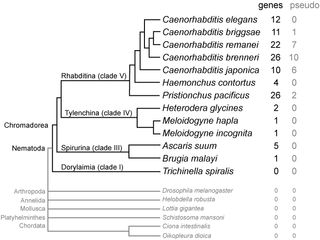
SmY ribonucleic acids are a family of small nuclear RNAs found in some species of nematode worms. They are thought to be involved in mRNA trans-splicing.
mRNA surveillance mechanisms are pathways utilized by organisms to ensure fidelity and quality of messenger RNA (mRNA) molecules. There are a number of surveillance mechanisms present within cells. These mechanisms function at various steps of the mRNA biogenesis pathway to detect and degrade transcripts that have not properly been processed.
Circular RNA is a type of single-stranded RNA which, unlike linear RNA, forms a covalently closed continuous loop. In circular RNA, the 3' and 5' ends normally present in an RNA molecule have been joined together. This feature confers numerous properties to circular RNA, many of which have only recently been identified.
Exitrons are produced through alternative splicing and have characteristics of both introns and exons, but are described as retained introns. Even though they are considered introns, which are typically cut out of pre mRNA sequences, there are significant problems that arise when exitrons are spliced out of these strands, with the most obvious result being altered protein structures and functions. They were first discovered in plants, but have recently been found in metazoan species as well.
The Shapiro–Senapathy algorithm (S&S) is an algorithm for predicting the splice sites, exons and genes in animals and plants. This algorithm has the ability to discover disease-causing mutations in splice junctions in cancerous and non-cancerous diseases that is being used in major research institutions around the world.
The split gene theory is a theory of the origin of introns, long non-coding sequences in eukaryotic genes between the exons. The theory holds that the randomness of primordial DNA sequences would only permit small (< 600bp) open reading frames (ORF), and that important intron structures and regulatory sequences are derived from stop codons. In this introns-first framework, the spliceosomal machinery and the nucleus evolved due to the necessity to join these ORFs into larger proteins, and that intronless bacterial genes are less ancestral than the split eukaryotic genes. The theory originated with Periannan Senapathy.
References
- ↑ Conrad, Richard; Fen Liou, Ruey; Blumenthal, Thomas (1993-02-25). "Functional analysis of a C. elegans trans-splice acceptor". Nucleic Acids Research. 21 (4): 913–919. doi:10.1093/nar/21.4.913. ISSN 0305-1048. PMC 309224 . PMID 8451190.
- 1 2 Stover, Nicholas A.; Kaye, Michelle S.; Cavalcanti, Andre R. O. (2006-01-10). "Spliced leader trans-splicing". Current Biology. 16 (1): R8–R9. doi: 10.1016/j.cub.2005.12.019 . ISSN 0960-9822. PMID 16401417.
- 1 2 3 Lasda, Erika L.; Blumenthal, Thomas (2011-05-01). "Trans-splicing". Wiley Interdisciplinary Reviews: RNA. 2 (3): 417–434. doi:10.1002/wrna.71. PMID 21957027. S2CID 209567118.
- ↑ "Oxford reference — Outron" . Retrieved 26 September 2019.
- ↑ "The MISO Sequence Ontology Browser — Outron (SO:0001475)" . Retrieved 26 September 2019.
- ↑ Clayton, Christine E. (2002-04-15). "Life without transcriptional control? From fly to man and back again". The EMBO Journal. 21 (8): 1881–1888. doi:10.1093/emboj/21.8.1881. ISSN 1460-2075. PMC 125970 . PMID 11953307.
- ↑ Blumenthal, Thomas; Gleason, Kathy Seggerson (February 2003). "Caenorhabditis elegans operons: form and function". Nature Reviews Genetics. 4 (2): 110–118. doi:10.1038/nrg995. ISSN 1471-0056. PMID 12560808. S2CID 9864778.
- ↑ Lei Q, Li C, Zuo Z, Huang C, Cheng H, Zhou R (March 2016). "Evolutionary Insights into RNA trans-Splicing in Vertebrates". Genome Biology and Evolution. 8 (3): 562–77. doi:10.1093/gbe/evw025. PMC 4824033 . PMID 26966239.