Elias James Corey is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.
In organic chemistry, the Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one of the many oxidation reactions commonly referred to as 'activated DMSO' oxidations. The reaction is known for its mild character and wide tolerance of functional groups.
Chromic acid is an inorganic acid composed of the elements chromium, oxygen, and hydrogen. It is a dark, purplish red, odorless, sand-like solid powder. When dissolved in water, it is a strong acid. There are 2 types of chromic acid: molecular chromic acid with the formula H
2CrO
4 and dichromic acid with the formula H
2Cr
2O
7.

Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula [C5H5NH]+[CrO3Cl]−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective.

Dess–Martin periodinane (DMP) is a chemical reagent used in the Dess–Martin oxidation, oxidizing primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions, shorter reaction times, higher yields, simplified workups, high chemoselectivity, tolerance of sensitive functional groups, and a long shelf life. However, use on an industrial scale is made difficult by its cost and its potentially explosive nature. It is named after the American chemists Daniel Benjamin Dess and James Cullen Martin who developed the reagent in 1983. It is based on IBX, but due to the acetate groups attached to the central iodine atom, DMP is much more reactive than IBX and is much more soluble in organic solvents.

The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis.

The Sarett oxidation is an organic reaction that oxidizes primary and secondary alcohols to aldehydes and ketones, respectively, using chromium trioxide and pyridine. Unlike the similar Jones oxidation, the Sarett oxidation will not further oxidize primary alcohols to their carboxylic acid form, neither will it affect carbon-carbon double bonds. Use of the original Sarett oxidation has become largely antiquated however, in favor of other modified oxidation techniques. The unadulterated reaction is still occasionally used in teaching settings and in small scale laboratory research.
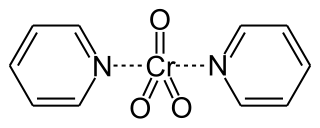
Collins reagent is the complex of chromium(VI) oxide with pyridine in dichloromethane. This metal-pyridine complex, a red solid, is used to oxidize primary alcohols to the corresponding aldehydes and secondary alcohols to the corresponding ketones. This complex is a hygroscopic orange solid.

The pyridinium dichromate(PDC) or Cornforth reagent is a pyridinium salt of dichromate with the chemical formula [C5H5NH]2[Cr2O7]. This compound is named after the Australian-British chemist Sir John Warcup Cornforth (b. 1917) who introduced it in 1962. The Cornforth reagent is a strong oxidizing agent which can convert primary and secondary alcohols to aldehydes and ketones respectively. In its chemical structure and functions it is closely related to other compounds made from hexavalent chromium oxide, such as pyridinium chlorochromate and Collins reagent. Because of their toxicity, these reagents are rarely used nowadays.
The oxidation of alcohols to carbonyls is an important family of oxidation reactions in organic chemistry. The reaction applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids. The reaction can occur using a variety of oxidants. In principle, a simple way to oxidatize an alcohols uses an oxygen atom and produce water:
Alcohol oxidation is a class of organic reactions in which the alcohol functional group is converted into another functional group in which carbon carries a higher oxidation state.

Oxoammonium-catalyzed oxidation reactions involve the conversion of organic substrates to more highly oxidized materials through the action of an N-oxoammonium species. Nitroxides may also be used in catalytic amounts in the presence of a stoichiometric amount of a terminal oxidant. Nitroxide radical species used are either 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or derivatives thereof.
Reactions of organocopper reagents involve species containing copper-carbon bonds acting as nucleophiles in the presence of organic electrophiles. Organocopper reagents are now commonly used in organic synthesis as mild, selective nucleophiles for substitution and conjugate addition reactions.
Heteroatom-promoted lateral lithiation is the site-selective replacement of a benzylic hydrogen atom for lithium for the purpose of further functionalization. Heteroatom-containing substituents may direct metalation to the benzylic site closest to the heteroatom or increase the acidity of the ring carbons via an inductive effect.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
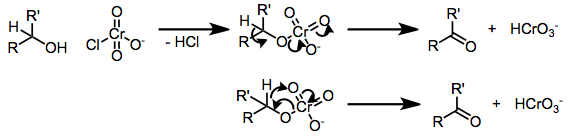
A chromate ester is a chemical structure that contains a chromium atom (symbol Cr) in a +6 oxidation state that is connected via an oxygen (O) linkage to a carbon (C) atom. The Cr itself is in its chromate form, with several oxygens attached, and the Cr–O–C attachment makes this chemical group structurally similar to other ester functional groups. They can be synthesized from various chromium(VI) metal compounds, such as CrO3, chromium chloride complexes, and aqueous chromate ions, and tend to react via redox reactions to liberate chromium(IV).
The Kröhnke pyridine synthesis is reaction in organic synthesis between α-pyridinium methyl ketone salts and α, β-unsaturated carbonyl compounds used to generate highly functionalized pyridines. Pyridines occur widely in natural and synthetic products, so there is wide interest in routes for their synthesis. The method is named after Fritz Kröhnke.

The Collins oxidation is an organic reaction for the oxidation of primary alcohols to aldehydes. It is distinguished from other chromium oxide-based oxidations by the use of Collins reagent, a complex of chromium(VI) oxide with pyridine in dichloromethane.
Sulfonium-based oxidations of alcohols to aldehydes summarizes a group of organic reactions that transform a primary alcohol to the corresponding aldehyde (and a secondary alcohol to the corresponding ketone). Selective oxidation of alcohols to aldehydes requires circumventing over-oxidation to the carboxylic acid. One popular approach are methods that proceed through intermediate alkoxysulfonium species (RO−SMe+
2X-, e.g. compound 6) as detailed here. Since most of these methods employ dimethylsulfoxide (DMSO) as oxidant and generate dimethylsulfide, these are often colloquially summarized as DMSO-oxidations. Conceptually, generating an aldehyde and dimethylsulfide from an alcohol and DMSO requires a dehydrating agent for removal of H2O, ideally an electrophile simultaneously activating DMSO. In contrast, methods generating the sulfonium intermediate from dimethylsulfide do not require a dehydrating agent. Closely related are oxidations mediated by dimethyl selenoxide and by dimethyl selenide.

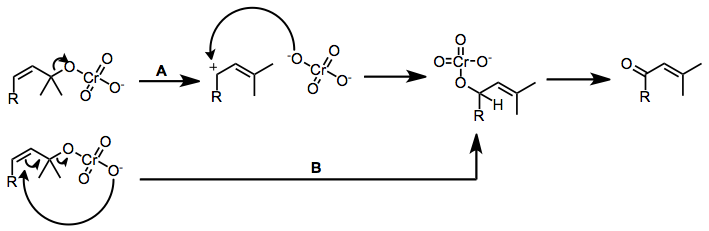
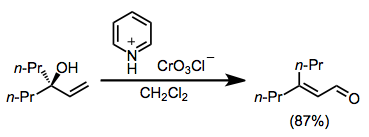
The Babler oxidation, also known as the Babler-Dauben oxidation, is an organic reaction for the oxidative transposition of tertiary allylic alcohols to enones using pyridinium chlorochromate (PCC):