Related Research Articles

Exocytosis is a form of active transport and bulk transport in which a cell transports molecules out of the cell. As an active transport mechanism, exocytosis requires the use of energy to transport material. Exocytosis and its counterpart, endocytosis, are used by all cells because most chemical substances important to them are large polar molecules that cannot pass through the hydrophobic portion of the cell membrane by passive means. Exocytosis is the process by which a large amount of molecules are released; thus it is a form of bulk transport. Exocytosis occurs via secretory portals at the cell plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structure at the cell plasma membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.

Auxins are a class of plant hormones with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essential for plant body development. The Dutch biologist Frits Warmolt Went first described auxins and their role in plant growth in the 1920s. Kenneth V. Thimann became the first to isolate one of these phytohormones and to determine its chemical structure as indole-3-acetic acid (IAA). Went and Thimann co-authored a book on plant hormones, Phytohormones, in 1937.
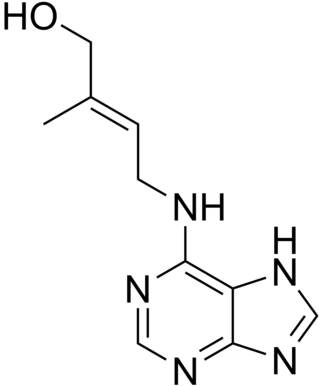
Cytokinins (CK) are a class of plant hormones that promote cell division, or cytokinesis, in plant roots and shoots. They are involved primarily in cell growth and differentiation, but also affect apical dominance, axillary bud growth, and leaf senescence.
Gibberellins (GAs) are plant hormones that regulate various developmental processes, including stem elongation, germination, dormancy, flowering, flower development, and leaf and fruit senescence. GAs are one of the longest-known classes of plant hormone. It is thought that the selective breeding of crop strains that were deficient in GA synthesis was one of the key drivers of the "green revolution" in the 1960s, a revolution that is credited to have saved over a billion lives worldwide.

In plant biology, thigmotropism is a directional growth movement which occurs as a mechanosensory response to a touch stimulus. Thigmotropism is typically found in twining plants and tendrils, however plant biologists have also found thigmotropic responses in flowering plants and fungi. This behavior occurs due to unilateral growth inhibition. That is, the growth rate on the side of the stem which is being touched is slower than on the side opposite the touch. The resultant growth pattern is to attach and sometimes curl around the object which is touching the plant. However, flowering plants have also been observed to move or grow their sex organs toward a pollinator that lands on the flower, as in Portulaca grandiflora.

Hydrotropism is a plant's growth response in which the direction of growth is determined by a stimulus or gradient in water concentration. A common example is a plant root growing in humid air bending toward a higher relative humidity level.

The dopamine transporter (DAT) also is a membrane-spanning protein coded for in the human by the SLC6A3 gene,, that pumps the neurotransmitter dopamine out of the synaptic cleft back into cytosol. In the cytosol, other transporters sequester the dopamine into vesicles for storage and later release. Dopamine reuptake via DAT provides the primary mechanism through which dopamine is cleared from synapses, although there may be an exception in the prefrontal cortex, where evidence points to a possibly larger role of the norepinephrine transporter.
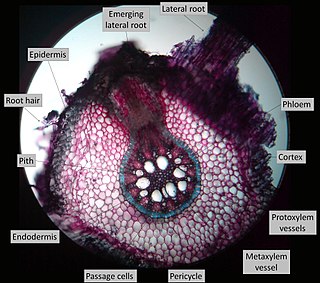
Lateral roots, emerging from the pericycle, extend horizontally from the primary root (radicle) and over time makeup the iconic branching pattern of root systems. They contribute to anchoring the plant securely into the soil, increasing water uptake, and facilitate the extraction of nutrients required for the growth and development of the plant. Lateral roots increase the surface area of a plant's root system and can be found in great abundance in several plant species. In some cases, lateral roots have been found to form symbiotic relationships with rhizobia (bacteria) and mycorrhizae (fungi) found in the soil, to further increase surface area and increase nutrient uptake.
Phospholipase D (EC 3.1.4.4, lipophosphodiesterase II, lecithinase D, choline phosphatase, PLD; systematic name phosphatidylcholine phosphatidohydrolase) is an enzyme of the phospholipase superfamily that catalyses the following reaction

Epsins are a family of highly conserved membrane proteins that are important in creating membrane curvature. Epsins contribute to membrane deformations like endocytosis, and block vesicle formation during mitosis.
Polar auxin transport is the regulated transport of the plant hormone auxin in plants. It is an active process, the hormone is transported in cell-to-cell manner and one of the main features of the transport is its asymmetry and directionality (polarity). The polar auxin transport functions to coordinate plant development; the following spatial auxin distribution underpins most of plant growth responses to its environment and plant growth and developmental changes in general. In other words, the flow and relative concentrations of auxin informs each plant cell where it is located and therefore what it should do or become.
Reticulons are a group of evolutionary conservative proteins residing predominantly in endoplasmic reticulum, primarily playing a role in promoting membrane curvature. In addition, reticulons may play a role in nuclear pore complex formation, vesicle formation, and other processes yet to be defined. They have also been linked to oligodendrocyte roles in inhibition of neurite outgrowth. Some studies link RTNs with Alzheimer's disease and amyotrophic lateral sclerosis.
Stp4 is a gene from the model plant, Arabidopsis thaliana. The gene transcribes for an integral membrane protein that is situated in the plasma membrane of sink tissues such as roots, anthers and vascular tissue.

In biology, phototropism is the growth of an organism in response to a light stimulus. Phototropism is most often observed in plants, but can also occur in other organisms such as fungi. The cells on the plant that are farthest from the light contain a hormone called auxin that reacts when phototropism occurs. This causes the plant to have elongated cells on the furthest side from the light. Phototropism is one of the many plant tropisms, or movements, which respond to external stimuli. Growth towards a light source is called positive phototropism, while growth away from light is called negative phototropism. Negative phototropism is not to be confused with skototropism, which is defined as the growth towards darkness, whereas negative phototropism can refer to either the growth away from a light source or towards the darkness. Most plant shoots exhibit positive phototropism, and rearrange their chloroplasts in the leaves to maximize photosynthetic energy and promote growth. Some vine shoot tips exhibit negative phototropism, which allows them to grow towards dark, solid objects and climb them. The combination of phototropism and gravitropism allow plants to grow in the correct direction.
In molecular biology, the auxin binding protein family is a family of proteins which bind the plant hormone auxin. They are located in the lumen of the endoplasmic reticulum (ER). The primary structure of these proteins contains an N-terminal hydrophobic leader sequence of 30-40 amino acids, which could represent a signal for translocation of the protein to the ER. The mature protein comprises around 165 residues, and contains a number of potential N-glycosylation sites. In vitro transport studies have demonstrated co-translational glycosylation. Retention within the lumen of the ER correlates with an additional signal located at the C terminus, represented by the sequence Lys-Asp-Glu-Leu, known to be responsible for preventing secretion of proteins from the lumen of the ER in eukaryotic cells.
The anion exchanger family is a member of the large APC superfamily of secondary carriers. Members of the AE family are generally responsible for the transport of anions across cellular barriers, although their functions may vary. All of them exchange bicarbonate. Characterized protein members of the AE family are found in plants, animals, insects and yeast. Uncharacterized AE homologues may be present in bacteria. Animal AE proteins consist of homodimeric complexes of integral membrane proteins that vary in size from about 900 amino acyl residues to about 1250 residues. Their N-terminal hydrophilic domains may interact with cytoskeletal proteins and therefore play a cell structural role. Some of the currently characterized members of the AE family can be found in the Transporter Classification Database.
The potassium (K+) uptake permease (KUP) family (TC# 2.A.72) is a member of the APC superfamily of secondary carriers. Proteins of the KUP/HAK/KT family include the KUP (TrkD) protein of E. coli and homologues in both Gram-positive and Gram-negative bacteria. High affinity (20 μM) K+ uptake systems (Hak1, TC# 2.A.72.2.1) of the yeast Debaryomyces occidentalis as well as the fungus, Neurospora crassa, and several homologues in plants have been characterized. Arabidopsis thaliana and other plants possess multiple KUP family paralogues. While many plant proteins cluster tightly together, the Hak1 proteins from yeast as well as the two Gram-positive and Gram-negative bacterial proteins are distantly related on the phylogenetic tree for the KUP family. All currently classified members of the KUP family can be found in the Transporter Classification Database.
As a model organism, the Arabidopsis thaliana response to salinity is studied to aid understanding of other more economically important crops.
Niko Geldner is a German-Swiss biologist specialised in the study of Plant Cell and Developmental Biology. He is a full professor and the director of the plant cell biology laboratory at the University of Lausanne.
The arrestin family of proteins is subdivided into α-arrestins (also referred to as arrestin-related trafficking adaptors or arrestin-like yeast proteins in yeast or ARRDCs in mammals, β-arrestins and Vps26-like arrestins proteins. The α-Arrestins are an ancestral branch of the larger arrestin family of proteins and they are conserved across eukaryotes but are best characterized in the budding yeast Saccharomyces cerevisiae; to-date there are 6 α-arrestins identified in mammalian cells and 14 α-arrestins identified in the budding yeast Saccharomyces cerevisiae. The yeast α-arrestin family comprises Ldb19/Art1, Ecm21/Art2, Aly1/Art6, Aly2/Art3, Rod1/Art4, Rog3/Art7, Art5, Csr2/Art8, Rim8/Art9, Art10, Bul1, Bul2, Bul3 and Spo23. The best characterized α-arrestin function to date is their endocytic regulation of plasma membrane proteins, including G-protein coupled receptors and nutrient transporters. α-Arrestins control endocytosis of these membrane proteins in response to cellular stressors, including nutrient or metal ion excess.
References
- ↑ Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, et al. (January 2005). "The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots". Nature. 433 (7021): 39–44. Bibcode:2005Natur.433...39B. doi:10.1038/nature03184. hdl: 1874/21119 . PMID 15635403. S2CID 4414301.
- ↑ Krecek P, Skupa P, Libus J, Naramoto S, Tejos R, Friml J, Zazímalová E (2009). "The PIN-FORMED (PIN) protein family of auxin transporters". Genome Biology. 10 (12): 249. doi: 10.1186/gb-2009-10-12-249 . PMC 2812941 . PMID 20053306.
- ↑ Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (July 1991). "Requirement of the Auxin Polar Transport System in Early Stages of Arabidopsis Floral Bud Formation". The Plant Cell. 3 (7): 677–684. doi:10.1105/tpc.3.7.677. PMC 160035 . PMID 12324609.
- ↑ Adamowski M, Friml J (January 2015). "PIN-dependent auxin transport: action, regulation, and evolution". The Plant Cell. 27 (1): 20–32. doi:10.1105/tpc.114.134874. PMC 4330589 . PMID 25604445.
- ↑ Jaillais Y, Ott T (April 2020). "The Nanoscale Organization of the Plasma Membrane and Its Importance in Signaling: A Proteolipid Perspective". Plant Physiology. 182 (4): 1682–1696. doi:10.1104/pp.19.01349. PMC 7140965 . PMID 31857424.
- ↑ Ke M, Ma Z, Wang D, Sun Y, Wen C, Huang D, et al. (January 2021). "Salicylic acid regulates PIN2 auxin transporter hyperclustering and root gravitropic growth via Remorin-dependent lipid nanodomain organisation in Arabidopsis thaliana". The New Phytologist. 229 (2): 963–978. doi:10.1111/nph.16915. PMC 7821329 . PMID 32901934.
- ↑ Feraru E, Feraru MI, Kleine-Vehn J, Martinière A, Mouille G, Vanneste S, et al. (February 2011). "PIN polarity maintenance by the cell wall in Arabidopsis". Current Biology. 21 (4): 338–343. doi: 10.1016/j.cub.2011.01.036 . PMID 21315597. S2CID 2117627.
- ↑ Li H, von Wangenheim D, Zhang X, Tan S, Darwish-Miranda N, Naramoto S, et al. (January 2021). "Cellular requirements for PIN polar cargo clustering in Arabidopsis thaliana". The New Phytologist. 229 (1): 351–369. doi:10.1111/nph.16887. PMC 7984064 . PMID 32810889.
- ↑ Narasimhan M, Gallei M, Tan S, Johnson A, Verstraeten I, Li L, et al. (June 2021). "Systematic analysis of specific and nonspecific auxin effects on endocytosis and trafficking". Plant Physiology. 186 (2): 1122–1142. doi:10.1093/plphys/kiab134. PMC 8195513 . PMID 33734402.
- ↑ Marhava P (January 2022). "Recent developments in the understanding of PIN polarity". The New Phytologist. 233 (2): 624–630. doi: 10.1111/nph.17867 . PMID 34882802. S2CID 245012549.
- ↑ Han H, Verstraeten I, Roosjen M, Mazur E, Rýdza N, Hajný J, et al. (2021). Rapid auxin-mediated phosphorylation of Myosin regulates trafficking and polarity in Arabidopsis (Report). doi:10.1101/2021.04.13.439603.