Related Research Articles

Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them.

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes.
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of palladium-catalyzed cross-couplings in organic synthesis. This reaction is also known as the Suzuki–Miyaura reaction or simply as the Suzuki coupling. It is widely used to synthesize polyolefins, styrenes, and substituted biphenyls. Several reviews have been published describing advancements and the development of the Suzuki reaction. The general scheme for the Suzuki reaction is shown below, where a carbon-carbon single bond is formed by coupling a halide (R1-X) with an organoboron species (R2-BY2) using a palladium catalyst and a base. The organoboron species is usually synthesized by hydroboration or carboboration, allowing for rapid generation of molecular complexity.
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide.

Raney nickel, also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable slurries. Raney nickel is used as a reagent and as a catalyst in organic chemistry. It was developed in 1926 by American engineer Murray Raney for the hydrogenation of vegetable oils. Raney is a registered trademark of W. R. Grace and Company. Other major producers are Evonik and Johnson Matthey.
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions.
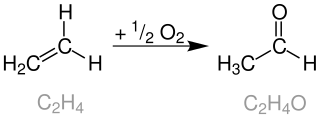
The Wacker process or the Hoechst-Wacker process refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride and copper(II) chloride as the catalyst. This chemical reaction was one of the first homogeneous catalysis with organopalladium chemistry applied on an industrial scale.
The Hiyama coupling is a palladium-catalyzed cross-coupling reaction of organosilanes with organic halides used in organic chemistry to form carbon–carbon bonds. This reaction was discovered in 1988 by Tamejiro Hiyama and Yasuo Hatanaka as a method to form carbon-carbon bonds synthetically with chemo- and regioselectivity. The Hiyama coupling has been applied to the synthesis of various natural products.
Adams' catalyst, also known as platinum dioxide, is usually represented as platinum(IV) oxide hydrate, PtO2•H2O. It is a catalyst for hydrogenation and hydrogenolysis in organic synthesis. This dark brown powder is commercially available. The oxide itself is not an active catalyst, but it becomes active after exposure to hydrogen whereupon it converts to platinum black, which is responsible for reactions.
Nanomaterial-based catalysts are usually heterogeneous catalysts broken up into metal nanoparticles in order to enhance the catalytic process. Metal nanoparticles have high surface area, which can increase catalytic activity. Nanoparticle catalysts can be easily separated and recycled. They are typically used under mild conditions to prevent decomposition of the nanoparticles.
Palladium on carbon, often referred to as Pd/C, is a form of palladium used as a catalyst. The metal is supported on activated carbon to maximize its surface area and activity.
Platinum on carbon, often referred to as Pt/C, is a form of platinum used as a catalyst. The metal is supported on activated carbon in order to maximize its surface area and activity.
In organic chemistry, the Buchwald–Hartwig amination is a chemical reaction for the synthesis of carbon–nitrogen bonds via the palladium-catalyzed coupling reactions of amines with aryl halides. Although Pd-catalyzed C-N couplings were reported as early as 1983, Stephen L. Buchwald and John F. Hartwig have been credited, whose publications between 1994 and the late 2000s established the scope of the transformation. The reaction's synthetic utility stems primarily from the shortcomings of typical methods for the synthesis of aromatic C−N bonds, with most methods suffering from limited substrate scope and functional group tolerance. The development of the Buchwald–Hartwig reaction allowed for the facile synthesis of aryl amines, replacing to an extent harsher methods while significantly expanding the repertoire of possible C−N bond formation.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent.
The Tsuji–Trost reaction is a palladium-catalysed substitution reaction involving a substrate that contains a leaving group in an allylic position. The palladium catalyst first coordinates with the allyl group and then undergoes oxidative addition, forming the π-allyl complex. This allyl complex can then be attacked by a nucleophile, resulting in the substituted product.
Urushibara nickel is a nickel based hydrogenation catalyst, named after Yoshiyuki Urushibara.
Rhodium-platinum oxide , or Nishimura's catalyst, is an inorganic compound used as a hydrogenation catalyst.
References
- 1 2 Nishimura, Shigeo (2001). Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (1st ed.). New York: Wiley-Interscience. pp. 34–35. ISBN 9780471396987.
- ↑ Iwasawa, Tetsuo; Tokunaga, Makoto; Obora, Yasushi; Tsuji, Yasushi (2004-06-01). "Homogeneous Palladium Catalyst Suppressing Pd Black Formation in Air Oxidation of Alcohols". Journal of the American Chemical Society. 126 (21): 6554–6555. doi:10.1021/ja031936l. ISSN 0002-7863. PMID 15161274.
- ↑ Nishimura, Shigeo; Itaya, Takashi; Shiota, Michio (1967). "Reactions of cycloalkanones in the presence of platinum-metal catalysts and hydrogen". Chemical Communications (9): 422–423. doi:10.1039/C19670000422.
- ↑ Zelinsky, N.; Glinka, N. (1911). "Über gleichzeitige Reduktions- und Oxydationskatalyse". Berichte der Deutschen Chemischen Gesellschaft. 44 (3): 2305–2311. doi:10.1002/cber.19110440347.