
Trichuriasis, also known as whipworm infection, is an infection by the parasitic worm Trichuris trichiura (whipworm). If infection is only with a few worms, there are often no symptoms. In those who are infected with many worms, there may be abdominal pain, fatigue and diarrhea. The diarrhea sometimes contains blood. Infections in children may cause poor intellectual and physical development. Low red blood cell levels may occur due to loss of blood.

Loa loa filariasis is a skin and eye disease caused by the nematode worm Loa loa. Humans contract this disease through the bite of a deer fly or mango fly, the vectors for Loa loa. The adult Loa loa filarial worm migrates throughout the subcutaneous tissues of humans, occasionally crossing into subconjunctival tissues of the eye where it can be easily observed. Loa loa does not normally affect one's vision but can be painful when moving about the eyeball or across the bridge of the nose. The disease can cause red itchy swellings below the skin called "Calabar swellings". The disease is treated with the drug diethylcarbamazine (DEC), and when appropriate, surgical methods may be employed to remove adult worms from the conjunctiva. Loiasis belongs to the so-called neglected diseases.

Loa loa is a filarial (arthropod-borne) nematode (roundworm) that causes Loa loa filariasis. Loa loa actually means "worm worm", but is commonly known as the "eye worm", as it localizes to the conjunctiva of the eye. Loa loa is commonly found in Africa. It mainly inhabits rain forests in West Africa and has native origins in Ethiopia. The disease caused by Loa loa is called loiasis and is one of the neglected tropical diseases.

Chronic wasting disease (CWD), sometimes called zombie deer disease, is a transmissible spongiform encephalopathy (TSE) affecting deer. TSEs are a family of diseases thought to be caused by misfolded proteins called prions and include similar diseases such as BSE in cattle, Creutzfeldt–Jakob disease (CJD) in humans and scrapie in sheep. Natural infection causing CWD affects members of the deer family. In the United States, CWD affects mule deer, white-tailed deer, red deer, sika deer, elk, caribou, and moose. Experimental transmission of CWD to other species such as squirrel monkeys and genetically modified (humanized) mice has been shown.

Onchocerciasis, also known as river blindness, is a disease caused by infection with the parasitic worm Onchocerca volvulus. Symptoms include severe itching, bumps under the skin, and blindness. It is the second-most common cause of blindness due to infection, after trachoma.

Dirofilaria immitis, also known as heartworm or dog heartworm, is a parasitic roundworm that is a type of filarial worm, a small thread-like worm, and which causes dirofilariasis. It is spread from host to host through the bites of mosquitoes. Four genera of mosquitoes transmit dirofilariasis, Aedes, Culex, Anopheles, and Mansonia. The definitive host is the dog, but it can also infect cats, wolves, coyotes, jackals, foxes, ferrets, bears, seals, sea lions and, under rare circumstances, humans.

Trichinella is the genus of parasitic roundworms of the phylum Nematoda that cause trichinosis. Members of this genus are often called trichinella or trichina worms. A characteristic of Nematoda is the one-way digestive tract, with a pseudocoelom.

Dictyocaulus is a genus of nematode parasites of the bronchial tree of horses, sheep, goats, deer, and cattle. Dictyocaulus arnfieldi is the lungworm of horses, and Dictyocaulus viviparus is the lungworm affecting ruminants.
Elaeophora schneideri is a nematode which infests several mammalian hosts in North America. It is transmitted by horse-flies. Infection in the normal definitive hosts, mule deer or black-tailed deer, seldom produces clinical symptoms. In other hosts, such as sheep, elk, moose, and goats, infection with E. schneideri leads to elaeophorosis. Symptoms of elaeophorosis include necrosis of the muzzle, ears, and optic nerves; lack of coordination (ataxia); facial or lower limb dermatitis; horn deformities; blindness; and death.

Moose sickness is a neurological condition seen in the northern mixed-wood forests of central and eastern North America where moose distribution overlaps with that of white-tailed deer. The disease is characterized by an unsteady gait, stumbling, head held to one side, circling, staying in one location, and in severe cases, being unable to get up.
Muelleries capillaris, also known as the hair or goat lungworm, is one of the most economically important nematodes of small ruminants. Although normally non-pathogenic in sheep, the parasite causes a disease condition called muelleriosis in goats. Sheep and goats commonly become infected after accidentally ingesting M. capillaris infected snails or slugs, and the parasite produces eggs in the lungs of its host, causing life-threatening bronchopneumonia in serious cases.
Lungworms are parasitic nematode worms of the order Strongylida that infest the lungs of vertebrates. The name is used for a variety of different groups of nematodes, some of which also have other common names; what they have in common is that they migrate to their hosts' lungs or respiratory tracts, and cause bronchitis or pneumonia. The lungworm will gradually damage the airways or lung tissue by inciting an inflammatory reaction inside the tissue. Ultimately, the parasites survive and reproduce in the respiratory tissues. The category is thus more a descriptive than a precisely taxonomic one.

Toxocara cati, also known as the feline roundworm, is a parasite of cats and other felids. It is one of the most common nematodes of cats, infecting both wild and domestic felids worldwide. Adult worms are localised in the gut of the host. In adult cats, the infection – which is called toxocariasis – is usually asymptomatic. However, massive infection in juvenile cats can be fatal.

Thelaziasis is the term for infestation with parasitic nematodes of the genus Thelazia. The adults of all Thelazia species discovered so far inhabit the eyes and associated tissues of various mammal and bird hosts, including humans. Thelazia nematodes are often referred to as "eyeworms".
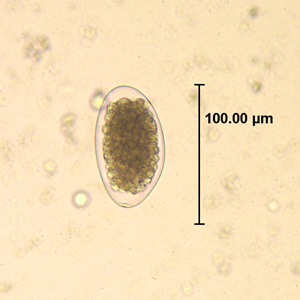
Trichostrongylus species are nematodes, which are ubiquitous among herbivores worldwide, including cattle, sheep, donkeys, goats, deer, and rabbits. At least 10 Trichostrongylus species have been associated with human infections. Infections occur via ingestion of infective larvae from contaminated vegetables or water. Epidemiological studies indicate a worldwide distribution of Trichostrongylus infections in humans, with the highest prevalence rates observed in individuals from regions with poor sanitary conditions, in rural areas, or who are farmers / herders. Human infections are most prevalent in the Middle East and Asia, with a worldwide estimated prevalence of 5.5 million people.

Baylisascaris procyonis, also known by the common name raccoon roundworm, is a roundworm nematode, found ubiquitously in raccoons, the definitive hosts. It is named after H. A. Baylis, who studied them in the 1920s–30s, and Greek askaris. Baylisascaris larvae in paratenic hosts can migrate, causing larva migrans. Baylisascariasis as the zoonotic infection of humans is rare, though extremely dangerous due to the ability of the parasite's larvae to migrate into brain tissue and cause damage. Concern for human infection has been increasing over the years due to the urbanization of rural areas, resulting in the increase in proximity and potential human interaction with raccoons.

Taenia hydatigena is one of the adult forms of the canine and feline tapeworm. This infection has a worldwide geographic distribution. Humans with taeniasis can infect other humans or animal intermediate hosts by eggs and gravid proglottids passed in the feces.

Cutaneous fibromas are common neoplasms occurring in wild and domestic deer of many species and are caused by host-specific viral infections. The fibromas occur most frequently in animals under 2 years of age, with cases in older deer reported occasionally or rarely.

Dermacentor albipictus, the winter tick, is a species of hard tick that parasitizes many different mammal species in North America. It is commonly associated with cervid species such as elk, white-tailed deer, mule deer and caribou but is primarily known as a serious pest of moose. As early as 1909, Ernest Thompson Seton described the winter tick as a greater enemy of the moose than were "wolves, bears, and cougars."
Setaria cervi is a species of parasitic roundworms belonging to the genus Setaria. It infects cattle, bison, yak, reindeer, buffalo, moose, and sheep all over the world. It is most prevalent in Europe and Asia. Different species of Aedes mosquito can transmit the filarial worm. Stable fly Haematobia stimulans is the major vector. The mature roundworms are primarily present in the abdominal (peritoneal) cavity, but are capable of migrating to central nervous system causing serious neurological disease.













