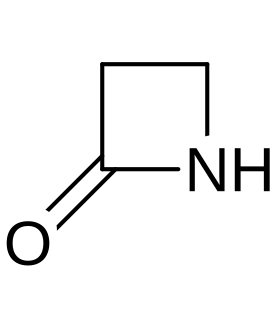
A beta-lactam (β-lactam) ring is a four-membered lactam. A lactam is a cyclic amide, and beta-lactams are named so because the nitrogen atom is attached to the β-carbon atom relative to the carbonyl. The simplest β-lactam possible is 2-azetidinone. β-lactams are significant structural units of medicines as manifested in many β-Lactam antibiotics Up to 1970, most β-lactam research was concerned with the penicillin and cephalosporin groups, but since then, a wide variety of structures have been described.

Penicillins are a group of antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens. Most penicillins in clinical use are chemically synthesised from naturally-produced penicillins. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G and penicillin V. Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are members of the β-lactam antibiotics. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use.
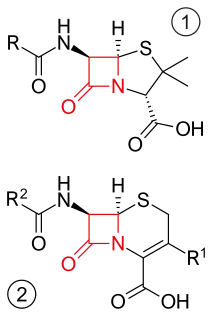
β-lactam antibiotics are antibiotics that contain a beta-lactam ring in their chemical structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems and carbacephems. Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds. The first β-lactam antibiotic discovered, penicillin, was isolated from a strain of Penicillium rubens.
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

Methicillin, also known as meticillin, is a narrow-spectrum β-lactam antibiotic of the penicillin class.

Anisomycin, also known as flagecidin, is an antibiotic produced by Streptomyces griseolus which inhibits eukaryotic protein synthesis. Partial inhibition of DNA synthesis occurs at anisomycin concentrations that effect 95% inhibition of protein synthesis. Anisomycin can activate stress-activated protein kinases, MAP kinase and other signal transduction pathways.
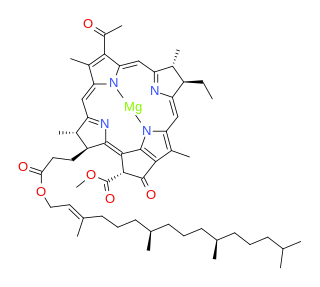
Bacteriochlorophylls (BChl) are photosynthetic pigments that occur in various phototrophic bacteria. They were discovered by C. B. van Niel in 1932. They are related to chlorophylls, which are the primary pigments in plants, algae, and cyanobacteria. Organisms that contain bacteriochlorophyll conduct photosynthesis to sustain their energy requirements, but do not produce oxygen as a byproduct. They use wavelengths of light not absorbed by plants or cyanobacteria. Replacement of Mg2+
with protons gives bacteriophaeophytin (BPh), the phaeophytin form.
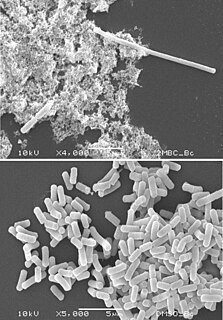
Filamentation, also termed conditional filamentation, is the anomalous growth of certain bacteria, such as Escherichia coli, in which cells continue to elongate but do not divide. The cells that result from elongation without division have multiple chromosomal copies. In the absence of antibiotics or other stressors, filamentation occurs at a low frequency in bacterial populations, the increased cell length protecting bacteria from protozoan predation and neutrophil phagocytosis by making ingestion of the cells more difficult. Filamentation is also thought to protect bacteria from antibiotics, and is associated with other aspects of bacterial virulence such as biofilm formation. The number and length of filaments within a bacterial population increases when the bacteria are treated with various chemical and physical agents. Some of the key genes involved in filamentation in E. coli include sulA and minCD.

A23187 is a mobile ion-carrier that forms stable complexes with divalent cations. A23187 is also known as Calcimycin, Calcium Ionophore, Antibiotic A23187 and Calcium Ionophore A23187. It is produced at fermentation of Streptomyceschartreusensis.

Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of enzymes called transpeptidases. Specifically, PBPs are DD-transpeptidases.

Prodigiosin is the red dyestuff produced by many strains of the bacterium Serratia marcescens, as well as other Gram-negative, gamma proteobacteria such as Vibrio psychroerythrus and Hahella chejuensis. It is responsible for the pink tint occasionally found in grime that accumulates on porcelain surfaces such as bathtubs, sinks, and toilet bowls. It is in the prodiginines family of compounds which are produced in some Gram-negative gamma proteobacteria, as well as select Gram-positive Actinobacteria. The name prodigiosin is derived from prodigious.
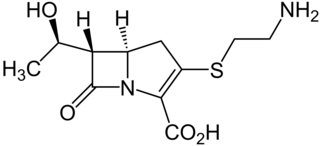
Thienamycin is one of the most potent naturally produced antibiotics known thus far, discovered in Streptomyces cattleya in 1976. Thienamycin has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase enzymes. Thienamycin is a zwitterion at pH 7.

Clavams are a class of antibiotics. This antibiotic is derived from Streptomyces clavuligerus NRRL 3585. Clavam is produced to form a new β-lactam antibiotic. This class is divided into the clavulanic acid class and the 5S clavams class. Clavulanic acid is a broad-spectrum antibiotic and 5S clavams may have anti-fungal properties. They are similar to penams, but with an oxygen substituted for the sulfur. Thus, they are also known as oxapenams.
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.
Cephalosporins are a broad class of bactericidal antibiotics that include the β-lactam ring and share a structural similarity and mechanism of action with other β-lactam antibiotics. The cephalosporins have the ability to kill bacteria by inhibiting essential steps in the bacterial cell wall synthesis which in the end results in osmotic lysis and death of the bacterial cell. Cephalosporins are widely used antibiotics because of their clinical efficiency and desirable safety profile.
Bacterial morphological plasticity refers to changes in the shape and size that bacterial cells undergo when they encounter stressful environments. Although bacteria have evolved complex molecular strategies to maintain their shape, many are able to alter their shape as a survival strategy in response to protist predators, antibiotics, the immune response, and other threats.
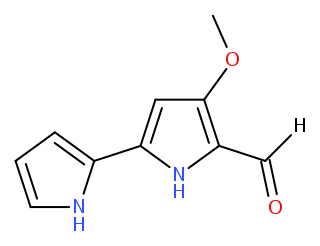
Tambjamines are a group of natural products that are structurally related to the prodiginines. They are enamine derivatives of 4-methoxy-2,2'-bipyrrole-5-carboxaldehyde (MBC).

Lipid II is a precursor molecule in the synthesis of the cell wall of bacteria. It is a peptidoglycan, which is amphipathic and named for its bactoprenol hydrocarbon chain, which acts as a lipid anchor, embedding itself in the bacterial cell membrane. Lipid II must translocate across the cell membrane to deliver and incorporate its disaccharide-pentapeptide "building block" into the peptidoglycan mesh. Lipid II is the target of several antibiotics.
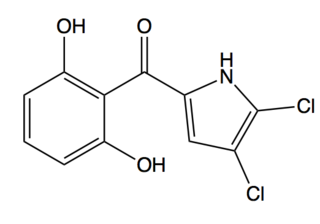
Pyoluteorin is a natural antibiotic that is biosynthesized from a hybrid nonribosomal peptide synthetase (NRPS) and polyketide synthase (PKS) pathway. Pyoluteorin was first isolated in the 1950s from Pseudomonas aeruginosa strains T359 and IFO 3455 and was found to be toxic against oomycetes, bacteria, fungi, and against certain plants. Pyoluteorin is most notable for its toxicity against the oomycete Pythium ultimum, which is a plant pathogen that causes a global loss in agriculture. Currently, pyoluteorin derivatives are being studied as an Mcl-1 antagonist in order to target cancers that have elevated Mcl-1 levels.

The prodiginines are a family of red tripyrrole dyestuffs produced by Gammaproteobacteria as well as some Actinomycetota. The group is named after prodigiosin (prodiginine) and is biosynthesized through a common set of enzymes. They are interesting due to their history and their varied biological activity.

















