In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.
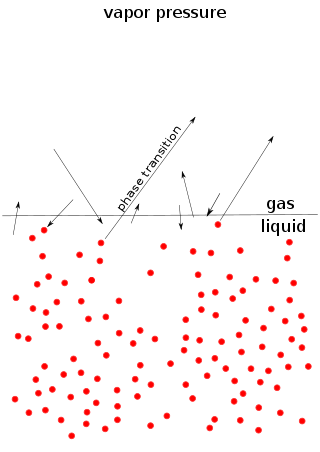
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions.
Hexane or n-hexane is an organic compound, a straight-chain alkane with six carbon atoms and the molecular formula C6H14.

In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. All cycloalkanes are isomers of alkenes.

In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached.
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without cleaving bonds. Usually hydrogenolysis is conducted catalytically using hydrogen gas.
Dodecane (also known as dihexyl, bihexyl, adakane 12, or duodecane) is an oily liquid n-alkane hydrocarbon with the chemical formula C12H26 (which has 355 isomers).
Tetradecane is an alkane hydrocarbon with the chemical formula CH3(CH2)12CH3.
Heptadecane is an organic compound, an alkane hydrocarbon with the chemical formula C17H36. The name may refer to any of 24894 theoretically possible structural isomers, or to a mixture thereof.

Tetracosane, also called tetrakosane, is an alkane hydrocarbon with the structural formula H(CH2)24H. As with other alkanes, its name is derived from Greek for the number of carbon atoms, 24, in the molecule. It has 14,490,245 constitutional isomers, and 252,260,276 stereoisomers.
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher pressure.

Beryllium fluoride is the inorganic compound with the formula Be F2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.

In organic chemistry, a bent bond, also known as a banana bond, is a type of covalent chemical bond with a geometry somewhat reminiscent of a banana. The term itself is a general representation of electron density or configuration resembling a similar "bent" structure within small ring molecules, such as cyclopropane (C3H6) or as a representation of double or triple bonds within a compound that is an alternative to the sigma and pi bond model.
In organic chemistry and organometallic chemistry, carbon–hydrogen bond activation is a type of organic reaction in which a carbon–hydrogen bond is cleaved and replaced with a C−X bond. Some authors further restrict the term C–H activation to reactions in which a C–H bond, one that is typically considered to be "unreactive", interacts with a transition metal center M, resulting in its cleavage and the generation of an organometallic species with an M–C bond. The intermediate of this step could then undergo subsequent reactions with other reagents, either in situ or in a separate step, to produce the functionalized product.
In gas chromatography, the Kovats retention index is used to convert retention times into system-independent constants. The index is named after the Hungarian-born Swiss chemist Ervin Kováts, who outlined the concept in the 1950s while performing research into the composition of the essential oils.

Pagodane is an organic compound with formula C
20H
20 whose carbon skeleton was said to resemble a pagoda, hence the name. It is a polycyclic hydrocarbon whose molecule has the D2h point symmetry group. The compound is a highly crystalline solid that melts at 243 °C, is barely soluble in most organic solvents and moderately soluble in benzene and chloroform. It sublimes at low pressure.
Oxidation with dioxiranes refers to the introduction of oxygen into organic molecules through the action of a dioxirane. Dioxiranes are well known for their oxidation of alkenes to epoxides; however, they are also able to oxidize other unsaturated functionality, heteroatoms, and alkane C-H bonds.
In organic chemistry, the hexadehydro-Diels–Alder (HDDA) reaction is an organic chemical reaction between a diyne and an alkyne to form a reactive benzyne species, via a [4+2] cycloaddition reaction. This benzyne intermediate then reacts with a suitable trapping agent to form a substituted aromatic product. This reaction is a derivative of the established Diels–Alder reaction and proceeds via a similar [4+2] cycloaddition mechanism. The HDDA reaction is particularly effective for forming heavily functionalized aromatic systems and multiple ring systems in one synthetic step.

1-Pentadecanol is an organic chemical compound classified as an alcohol. At room temperature, it is a white, flaky solid. It is a saturated long-chain fatty alcohol consisting of a pentadecane chain with a hydroxy group as substituent on one end. It is an achiral molecule.