
The halogens are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17.

Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης, meaning 'violet'.

The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. Haloarenes are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.

Hydrogen iodide (HI) is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.

Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt.

Zinc iodide is the inorganic compound with the formula ZnI2. It exists both in anhydrous form and as a dihydrate. Both are white and readily absorb water from the atmosphere. It has no major application.
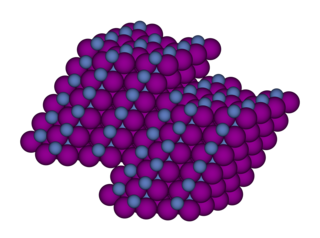
Nickel(II) iodide is an inorganic compound with the formula NiI2. This paramagnetic black solid dissolves readily in water to give bluish-green solutions, from which crystallizes the aquo complex [Ni(H2O)6]I2 (image above). This bluish-green colour is typical of hydrated nickel(II) compounds. Nickel iodides find some applications in homogeneous catalysis.

Iodine monochloride is an interhalogen compound with the formula ICl. It is a red-brown chemical compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, this molecule is highly polar and behaves as a source of I+. Discovered in 1814 by Gay-Lussac, iodine monochloride is the first interhalogen compound discovered.
Iodine compounds are compounds containing the element iodine. Iodine can form compounds using multiple oxidation states. Iodine is quite reactive, but it is much less reactive than the other halogens. For example, while chlorine gas will halogenate carbon monoxide, nitric oxide, and sulfur dioxide, iodine will not do so. Furthermore, iodination of metals tends to result in lower oxidation states than chlorination or bromination; for example, rhenium metal reacts with chlorine to form rhenium hexachloride, but with bromine it forms only rhenium pentabromide and iodine can achieve only rhenium tetraiodide. By the same token, however, since iodine has the lowest ionisation energy among the halogens and is the most easily oxidised of them, it has a more significant cationic chemistry and its higher oxidation states are rather more stable than those of bromine and chlorine, for example in iodine heptafluoride.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

Magnesium iodide is an inorganic compound with the chemical formula MgI2. It forms various hydrates MgI2·xH2O. Magnesium iodide is a salt of magnesium and hydrogen iodide. These salts are typical ionic halides, being highly soluble in water.

Beryllium iodide is an inorganic compound with the chemical formula BeI2. It is a hygroscopic white solid. The Be2+ cation, which is relevant to salt-like BeI2, is characterized by the highest known charge density (Z/r = 6.45), making it one of the hardest cations and a very strong Lewis acid.
Organoiodine chemistry is the study of the synthesis and properties of organoiodine compounds, or organoiodides, organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.
Iron(III) iodide is an inorganic compound with the chemical formula FeI3. It is a thermodynamically unstable compound that is difficult to prepare. Nevertheless, iron(III) iodide has been synthesised in small quantities in the absence of air and water.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.














