
Bacterial lawn is a term used by microbiologists to describe the appearance of bacterial colonies when all the individual colonies on a Petri dish agar plate merge to form a field or mat of bacteria. Bacterial lawns find use in screens for antibiotic resistance and bacteriophage titering.

A Petri dish is a shallow transparent lidded dish that biologists use to hold growth medium in which cells can be cultured, originally, cells of bacteria, fungi and small mosses. The container is named after its inventor, German bacteriologist Julius Richard Petri. It is the most common type of culture plate. The Petri dish is one of the most common items in biology laboratories and has entered popular culture. The term is sometimes written in lower case, especially in non-technical literature.

An agar plate is a Petri dish that contains a growth medium solidified with agar, used to culture microorganisms. Sometimes selective compounds are added to influence growth, such as antibiotics.

A microbiological culture, or microbial culture, is a method of multiplying microbial organisms by letting them reproduce in predetermined culture medium under controlled laboratory conditions. Microbial cultures are foundational and basic diagnostic methods used as research tools in molecular biology.

Bacteriological water analysis is a method of analysing water to estimate the numbers of bacteria present and, if needed, to find out what sort of bacteria they are. It represents one aspect of water quality. It is a microbiological analytical procedure which uses samples of water and from these samples determines the concentration of bacteria. It is then possible to draw inferences about the suitability of the water for use from these concentrations. This process is used, for example, to routinely confirm that water is safe for human consumption or that bathing and recreational waters are safe to use.

A blood culture is a medical laboratory test used to detect bacteria or fungi in a person's blood. Under normal conditions, the blood does not contain microorganisms: their presence can indicate a bloodstream infection such as bacteremia or fungemia, which in severe cases may result in sepsis. By culturing the blood, microbes can be identified and tested for resistance to antimicrobial drugs, which allows clinicians to provide an effective treatment.

Coliform bacteria are defined as either motile or non-motile Gram-negative non-spore forming bacilli that possess β-galactosidase to produce acids and gases under their optimal growth temperature of 35–37 °C. They can be aerobes or facultative aerobes, and are a commonly used indicator of low sanitary quality of foods, milk, and water. Coliforms can be found in the aquatic environment, in soil and on vegetation; they are universally present in large numbers in the feces of warm-blooded animals as they are known to inhabit the gastrointestinal system. While coliform bacteria are not normally causes of serious illness, they are easy to culture, and their presence is used to infer that other pathogenic organisms of fecal origin may be present in a sample, or that said sample is not safe to consume. Such pathogens include disease-causing bacteria, viruses, or protozoa and many multicellular parasites. Every drinking water source must be tested for the presence of these total coliform bacteria.

A growth medium or culture medium is a solid, liquid, or semi-solid designed to support the growth of a population of microorganisms or cells via the process of cell proliferation or small plants like the moss Physcomitrella patens. Different types of media are used for growing different types of cells.

Julius Richard Petri was a German microbiologist who is generally credited with inventing the device known as the Petri dish, which is named after him, while working as assistant to bacteriologist Robert Koch.
A fecal coliform is a facultatively anaerobic, rod-shaped, gram-negative, non-sporulating bacterium. Coliform bacteria generally originate in the intestines of warm-blooded animals. Fecal coliforms are capable of growth in the presence of bile salts or similar surface agents, are oxidase negative, and produce acid and gas from lactose within 48 hours at 44 ± 0.5°C. The term thermotolerant coliform is more correct and is gaining acceptance over "fecal coliform".

Haemophilus is a genus of Gram-negative, pleomorphic, coccobacilli bacteria belonging to the family Pasteurellaceae. While Haemophilus bacteria are typically small coccobacilli, they are categorized as pleomorphic bacteria because of the wide range of shapes they occasionally assume. These organisms inhabit the mucous membranes of the upper respiratory tract, mouth, vagina, and intestinal tract. The genus includes commensal organisms along with some significant pathogenic species such as H. influenzae—a cause of sepsis and bacterial meningitis in young children—and H. ducreyi, the causative agent of chancroid. All members are either aerobic or facultatively anaerobic. This genus has been found to be part of the salivary microbiome.
In microbiology, colony-forming unit is a unit which estimates the number of microbial cells in a sample that are viable, able to multiply via binary fission under the controlled conditions. Counting with colony-forming units requires culturing the microbes and counts only viable cells, in contrast with microscopic examination which counts all cells, living or dead. The visual appearance of a colony in a cell culture requires significant growth, and when counting colonies, it is uncertain if the colony arose from one cell or a group of cells. Expressing results as colony-forming units reflects this uncertainty.

Simmons' citrate agar is used for differentiating gram-negative bacteria on the basis of citrate utilization, especially for distinguishing Gammaproteobacteria of the family Enterobacteriaceae or even between species of the same genus. For example, Salmonella enteritidis would yield a positive (blue) result on Simmons’ agar and thus be distinguished from other Salmonella species like Salmonella typhi, Salmonella pullorum, and Salmonella gallinarum, which would yield a negative (green) result.
The Miles and Misra Method is a technique used in Microbiology to determine the number of colony forming units in a bacterial suspension or homogenate. The technique was first described in 1938 by Miles, Misra and Irwin who at the time were working at the LSHTM. The Miles and Misra method has been shown to be precise.
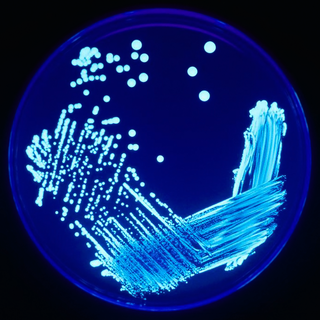
In microbiology, streaking is a technique used to isolate a pure strain from a single species of microorganism, often bacteria. Samples can then be taken from the resulting colonies and a microbiological culture can be grown on a new plate so that the organism can be identified, studied, or tested.
Total viable count (TVC), gives a quantitative estimate of the concentration of microorganisms such as bacteria, yeast or mould spores in a sample. The count represents the number of colony forming units (cfu) per g of the sample.
Plate count agar (PCA), also called standard methods agar (SMA), is a microbiological growth medium commonly used to assess or to monitor "total" or viable bacterial growth of a sample. PCA is not a selective medium.
Virtual colony count (VCC) is a kinetic, 96-well microbiological assay originally developed to measure the activity of defensins. It has since been applied to other antimicrobial peptides including LL-37. It utilizes a method of enumerating bacteria called quantitative growth kinetics, which compares the time taken for a bacterial batch culture to reach a threshold optical density with that of a series of calibration curves. The name VCC has also been used to describe the application of quantitative growth kinetics to enumerate bacteria in cell culture infection models. Antimicrobial susceptibility testing (AST) can be done on 96-well plates by diluting the antimicrobial agent at varying concentrations in broth inoculated with bacteria and measuring the minimum inhibitory concentration that results in no growth. However, these methods cannot be used to study some membrane-active antimicrobial peptides, which are inhibited by the broth itself. The virtual colony count procedure takes advantage of this fact by first exposing bacterial cells to the active antimicrobial agent in a low-salt buffer for two hours, then simultaneously inhibiting antimicrobial activity and inducing exponential growth by adding broth. The growth kinetics of surviving cells can then be monitored using a temperature-controlled plate reader. The time taken for each growth curve to reach a threshold change in optical density is then converted into virtual survival values, which serve as a measure of antimicrobial activity.

A spiral plater is an instrument used to dispense a liquid sample onto a Petri dish in a spiral pattern. Commonly used as part of a CFU count procedure for the purpose of determining the number of microbes in the sample. In this setting, after spiral plating, the Petri dish is incubated for several hours after which the number of colony forming microbes (CFU) is determined. Spiral platers are also used for research, clinical diagnostics and as a method for covering a Petri dish with bacteria before placing antibiotic discs for AST.
In microbiology, the term isolation refers to the separation of a strain from a natural, mixed population of living microbes, as present in the environment, for example in water or soil, or from living beings with skin flora, oral flora or gut flora, in order to identify the microbe(s) of interest. Historically, the laboratory techniques of isolation first developed in the field of bacteriology and parasitology, before those in virology during the 20th century.

















