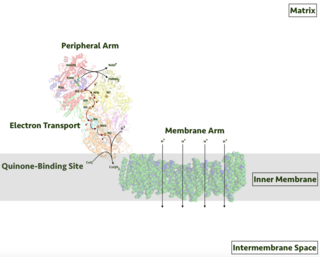
Respiratory complex I, EC 7.1.1.2 is the first large protein complex of the respiratory chains of many organisms from bacteria to humans. It catalyzes the transfer of electrons from NADH to coenzyme Q10 (CoQ10) and translocates protons across the inner mitochondrial membrane in eukaryotes or the plasma membrane of bacteria.

The coenzyme Q : cytochrome c – oxidoreductase, sometimes called the cytochrome bc1 complex, and at other times complex III, is the third complex in the electron transport chain, playing a critical role in biochemical generation of ATP. Complex III is a multisubunit transmembrane protein encoded by both the mitochondrial and the nuclear genomes. Complex III is present in the mitochondria of all animals and all aerobic eukaryotes and the inner membranes of most eubacteria. Mutations in Complex III cause exercise intolerance as well as multisystem disorders. The bc1 complex contains 11 subunits, 3 respiratory subunits, 2 core proteins and 6 low-molecular weight proteins.

Coenzyme Q is a coenzyme family that is ubiquitous in animals and many Pseudomonadota (hence its other name, ubiquinone). In humans, the most common form is coenzyme Q10 (which is also called CoQ10 and ubiquinone-10.

A dietary supplement is a manufactured product intended to supplement a person's diet by taking a pill, capsule, tablet, powder, or liquid. A supplement can provide nutrients either extracted from food sources, or that are synthetic. The classes of nutrient compounds in supplements include vitamins, minerals, fiber, fatty acids, and amino acids. Dietary supplements can also contain substances that have not been confirmed as being essential to life, and so are not nutrients per se, but are marketed as having a beneficial biological effect, such as plant pigments or polyphenols. Animals can also be a source of supplement ingredients, such as collagen from chickens or fish for example. These are also sold individually and in combination, and may be combined with nutrient ingredients. The European Commission has also established harmonized rules to help insure that food supplements are safe and appropriately labeled.

Current good manufacturing practices (cGMP) are those conforming to the guidelines recommended by relevant agencies. Those agencies control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. These guidelines provide minimum requirements that a manufacturer must meet to assure that their products are consistently high in quality, from batch to batch, for their intended use. The rules that govern each industry may differ significantly; however, the main purpose of GMP is always to prevent harm from occurring to the end user. Additional tenets include ensuring the end product is free from contamination, that it is consistent in its manufacture, that its manufacture has been well documented, that personnel are well trained, and that the product has been checked for quality more than just at the end phase. GMP is typically ensured through the effective use of a quality management system (QMS).

A nutraceutical is a pharmaceutical alternative that claims physiological benefits. In the US, nutraceuticals are largely unregulated, as they exist in the same category as dietary supplements and food additives by the FDA, under the authority of the Federal Food, Drug, and Cosmetic Act. The word "nutraceutical" is a portmanteau term, blending the words "nutrition" and "pharmaceutical".
The pharmaceutical industry is one of the leading industries in the People's Republic of China, covering synthetic chemicals and drugs, prepared Chinese medicines, medical devices, apparatus and instruments, hygiene materials, packing materials, and pharmaceutical machinery. China has the second-largest pharmaceutical market in the world as of 2017 which is worth US$110 billion. China accounts for 20% of the world's population but only a small fraction of the global drug market. China's changing health-care environment is designed to extend basic health insurance to a larger portion of the population and give individuals greater access to products and services. Following the period of change, the pharmaceutical industry is expected to continue its expansion.

Electron-transferring-flavoprotein dehydrogenase is an enzyme that transfers electrons from electron-transferring flavoprotein in the mitochondrial matrix, to the ubiquinone pool in the inner mitochondrial membrane. It is part of the electron transport chain. The enzyme is found in both prokaryotes and eukaryotes and contains a flavin and FE-S cluster. In humans, it is encoded by the ETFDH gene. Deficiency in ETF dehydrogenase causes the human genetic disease multiple acyl-CoA dehydrogenase deficiency.

MT-ND5 is a gene of the mitochondrial genome coding for the NADH-ubiquinone oxidoreductase chain 5 protein (ND5). The ND5 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. Variations in human MT-ND5 are associated with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) as well as some symptoms of Leigh's syndrome and Leber's hereditary optic neuropathy (LHON).
Stephen T. Sinatra (15 October 1946 –19 June 2022) was a board-certified cardiologist specializing in integrative medicine. He was also a certified bioenergetic psychotherapist. He has published journal articles on cholesterol and coenzyme Q10. He has appeared on national radio and television broadcasts, including The Dr. Oz Show, The Doctors, CNN’s “Sunday Morning News,” XM Radio’s “America’s Doctor Dr. Mehmet Oz,” and PBS’s “Body & Soul." He was also the author of the monthly newsletter Heart, Health & Nutrition and founder of Heart MD Institute. Sinatra died on June 19, 2022.
Pharmaceutical fraud involves activities that result in false claims to insurers or programs such as Medicare in the United States or equivalent state programs for financial gain to a pharmaceutical company. There are several different schemes used to defraud the health care system which are particular to the pharmaceutical industry. These include: Good Manufacturing Practice (GMP) Violations, Off Label Marketing, Best Price Fraud, CME Fraud, Medicaid Price Reporting, and Manufactured Compound Drugs. Examples of fraud cases include the GlaxoSmithKline $3 billion settlement, Pfizer $2.3 billion settlement, and Merck $650 million settlement. Damages from fraud can be recovered by use of the False Claims Act, most commonly under the qui tam provisions which rewards an individual for being a "whistleblower", or relator (law).
The Q-Symbio study was an international multi-center clinical trial that was reported in the Journal of the American College of Cardiology: Heart Failure in September 2014.

NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 5, 16kDa is a protein that in humans is encoded by the NDUFAB1 gene. The NDUFAB1 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain.
Bergstrom Nutrition is a United States dietary supplement manufacturer. As of June 2015, they are the only North American manufactures of methylsulfonylmethane (MSM), an ingredient utilized in dietary supplement products. Bergstrom Nutrition use proprietary technologies for the distillation and purification of MSM. This along with their extensive published safety data allowed their product to be self-affirmed as "Generally recognized as safe" (GRAS) receiving a letter of non-objection from the United States Food and Drug Administration.
Svend Aage Mortensen, M.D., Sc.D. was a Danish cardiologist at Rigshospitalet in Copenhagen, Denmark. Rigshospitalet was until February 2017 the largest hospital in Denmark; only surpassed by Skejby Sygehus, however, Rigshospitalet is a flagship in the Danish health care system. From 1990 until his death in 2015, Mortensen was the medical director of the heart transplantation unit at Rigshospitalet. He was a fellow of the European Society of Cardiologists. Mortensen is best known as the chief researcher and the leading author for the Q-Symbio study of the "Effect of Coenzyme Q10 on Morbidity and Mortality in Chronic Heart Failure."
Solanesol is the organic compound with the formula Me2C=CHCH2(CH2C(Me)=CHCH2)8OH. It is an all trans stereoisomer. This white, waxy solid is classified as an nonaisoprenoid. Solanesol is a non-cyclic terpene alcohol that consists of nine isoprene units and mainly accumulates in solanaceous plants such as tobacco, potato, and tomato. It is extractable from the stems and leaves of solanaceous species. It is notable as the biosynthetic precursor to coenzyme Q10.
William V. Judy, Ph.D. was an American author, clinical researcher, clinical trial consultant, and retired professor of physiology and biophysics. He was first introduced to the field of Coenzyme Q10 clinical research by Karl Folkers, the American bio-chemist who determined the structure of the Coenzyme Q10 molecule.

The International Coenzyme Q10 Association is a nonprofit association originally based in Ancona, Italy and currently in Seville, Spain. Since its establishment in 1997, it has promoted biochemical and clinical research on the substance Coenzyme Q10 in an attempt to increase the body of knowledge about the preventive and therapeutic health effects of Coenzyme Q10.

Plácido Navas Lloret is a Spanish Professor of Cell Biology in the Andalusian Center for Developmental Biology at the Pablo de Olavide University in Sevilla, Spain. From 2002 to 2012, Professor Navas served as a board member of the International Coenzyme Q10 Association; since 2013, he has been the chairman of the association.

Mitoquinone mesylate (MitoQ) is a synthetic analogue of coenzyme Q10 which has antioxidant effects. It was first developed in New Zealand in the late 1990s. It has significantly improved bioavailability and improved mitochondrial penetration compared to coenzyme Q10, and has shown potential in a number of medical indications, being widely sold as a dietary supplement.











