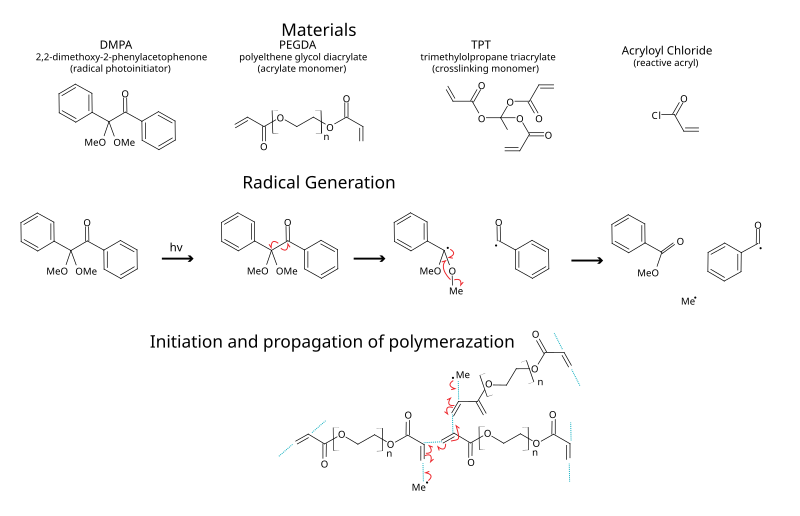
In chemistry, a photoinitiator is a molecule that creates reactive species (free radicals, cations or anions) when exposed to radiation (UV or visible). Synthetic photoinitiators are key components in photopolymers (for example, photo-curable coatings, adhesives and dental restoratives).
Contents
- Reactions
- Atmospheric photoinitiators
- Peroxides
- Nitrogen dioxide
- Molecular oxygen
- Commercial photoinitiators and uses
- AIBN
- Benzoyl peroxide
- 2,2-Dimethoxy-2-phenylacetophenone
- Camphorquinone
- Irgacure 819
- See also
- References
- Bibliography
Some small molecules in the atmosphere can also act as photoinitiators by decomposing to give free radicals (in photochemical smog). For instance, nitrogen dioxide (NO2) is produced in large quantities by gasoline-burning internal combustion engines. NO2 in the troposphere gives smog its brown coloration and catalyzes production of toxic ground-level ozone (O3). Molecular oxygen (O2) also serves as a photoinitiator in the stratosphere, breaking down into atomic oxygen and combining with O2 in order to form the ozone in the ozone layer.