
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution, and may require a mordant to improve the fastness of the dye on the fiber.

Ink is a gel, sol, or solution that contains at least one colourant, such as a dye or pigment, and is used to color a surface to produce an image, text, or design. Ink is used for drawing or writing with a pen, brush, reed pen, or quill. Thicker inks, in paste form, are used extensively in letterpress and lithographic printing.

A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compounds. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli.
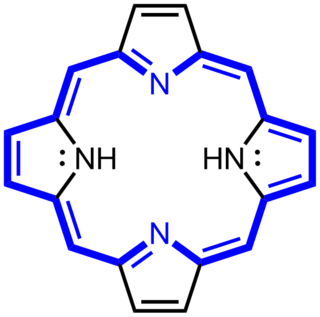
Porphyrins are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons, of which 18 π-electrons form a planar, continuous cycle, the porphyrin ring structure is often described as aromatic. One result of the large conjugated system is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives from the Greek word πορφύρα (porphyra), meaning purple.

Prussian blue is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula FeIII
4[FeII
(CN)
6]
3. Turnbull's blue is chemically identical, but is made from different reagents, and its slightly different color stems from different impurities.

In aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant.

Photodynamic therapy (PDT), is a form of phototherapy involving light and a photosensitizing chemical substance, used in conjunction with molecular oxygen to elicit cell death (phototoxicity).
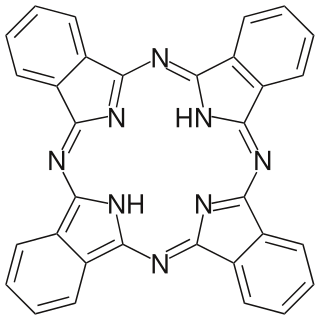
Phthalocyanine is a large, aromatic, macrocyclic, organic compound with the formula (C8H4N2)4H2 and is of theoretical or specialized interest in chemical dyes and photoelectricity.

Quinacridone is an organic compound used as a pigment. Numerous derivatives constitute the quinacridone pigment family, which finds extensive use in industrial colorant applications such as robust outdoor paints, inkjet printer ink, tattoo inks, artists' watercolor paints, and color laser printer toner. As pigments, the quinacridones are insoluble. The development of this family of pigments supplanted the alizarin dyes.

Thermochromism is the property of substances to change color due to a change in temperature. A mood ring is an excellent example of this phenomenon, but thermochromism also has more practical uses, such as baby bottles which change to a different color when cool enough to drink, or kettles which change when water is at or near boiling point. Thermochromism is one of several types of chromism.

Paris green is an inorganic compound. As a green pigment it is also known as Schweinfurt green, emerald green or Vienna green. It is a highly toxic emerald-green crystalline powder that has been used as a rodenticide and insecticide, and also as a pigment, despite its toxicity. It is also used as a blue colorant for fireworks. The color of Paris green is said to range from a pale blue green when very finely ground, to a deeper green when coarsely ground.

Phthalocyanine green G, which has many commercial names, is a synthetic green pigment from the group of phthalocyanine dyes, a complex of copper(II) with chlorinated phthalocyanine. It is a soft green powder, which is insoluble in water. It is a bright, high intensity colour used in oil and acrylic based artist's paints, and in other applications.

Han purple and Han blue are synthetic barium copper silicate pigments developed in China and used in ancient and imperial China from the Western Zhou period until the end of the Han dynasty.
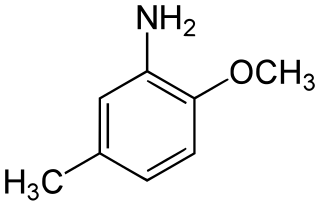
para-Cresidine is an organic compound with the formula CH3OC6H3(CH3)NH2. It is a white solid that is soluble in organic solvents. The compound features both amine and methoxy functional groups. It is used as an intermediate in preparation of dyes and pigments.

Alcian blue is any member of a family of polyvalent basic dyes, of which the Alcian blue 8G has been historically the most common and the most reliable member. It is used to stain acidic polysaccharides such as glycosaminoglycans in cartilages and other body structures, some types of mucopolysaccharides, sialylated glycocalyx of cells etc. For many of these targets it is one of the most widely used cationic dyes for both light and electron microscopy. Use of alcian blue has historically been a popular staining method in histology especially for light microscopy in paraffin embedded sections and in semithin resin sections. The tissue parts that specifically stain by this dye become blue to bluish-green after staining and are called "Alcianophilic". Alcian blue staining can be combined with H&E staining, PAS staining and van Gieson staining methods. Alcian blue can be used to quantitate acidic glycans both in microspectrophotometric quantitation in solution or for staining glycoproteins in polyacrylamide gels or on western blots. Biochemists had used it to assay acid polysaccharides in urine since the 1960s for diagnosis of diseases like mucopolysaccharidosis but from 1970's, partly due to lack of availability of Alcian and partly due to length and tediousness of the procedure, alternative methods had to be developed e.g. Dimethyl methylene blue method.

Phthalonitrile is an organic compound with the formula C6H4(CN)2, which is an off-white crystal solid at room temperature. It is a derivative of benzene, containing two adjacent nitrile groups. The compound has low solubility in water but is soluble in common organic solvents. The compound is used as a precursor to phthalocyanine and other pigments, fluorescent brighteners, and photographic sensitizers.

A rylene dye is a dye based on the rylene framework of naphthalene units linked in peri-positions. In homologues additional naphthalene units are added, forming compounds — or poly(peri-naphthalene)s — such as perylene, terrylene and quarterrylene.
In coordination chemistry, a macrocyclic ligand is a macrocyclic ring having at least nine atoms and three or more donor sites that serve as ligands that can bind to a central metal ion. Crown ethers and porphyrins are prominent examples. Macrocyclic ligands exhibit high affinity for metal ions.

YInMn Blue, also known as Oregon Blue or Mas Blue, is an inorganic blue pigment that was discovered accidentally by Professor Mas Subramanian and his (then) graduate student, Andrew E. Smith, at Oregon State University in 2009. The pigment is noteworthy for its vibrant, near-perfect blue color and unusually high NIR reflectance. The chemical compound has a unique crystal structure in which trivalent manganese ions in the trigonal bipyramidal coordination are responsible for the observed intense blue color. Since the initial discovery, the fundamental principles of colour science have been extensively explored by the Subramanian research team at Oregon State University, resulting in a wide range of rationally designed novel green, purple, and orange pigments, all through intentional addition of a chromophore in the trigonal bipyramidal coordination environment.

A colorant is any substance that changes the spectral transmittance or reflectance of a material. Synthetic colorants are those created in a laboratory or industrial setting. The production and improvement of colorants was a driver of the early synthetic chemical industry, in fact many of today's largest chemical producers started as dye-works in the late 19th or early 20th centuries, including Bayer AG(1863). Synthetics are extremely attractive for industrial and aesthetic purposes as they have they often achieve higher intensity and color fastness than comparable natural pigments and dyes used since ancient times. Market viable large scale production of dyes occurred nearly simultaneously in the early major producing countries Britain (1857), France (1858), Germany (1858), and Switzerland (1859), and expansion of associated chemical industries followed. The mid-nineteenth century through WWII saw an incredible expansion of the variety and scale of manufacture of synthetic colorants. Synthetic colorants quickly became ubiquitous in everyday life, from clothing to food. This stems from the invention of industrial research and development laboratories in the 1870s, and the new awareness of empirical chemical formulas as targets for synthesis by academic chemists. The dye industry became one of the first instances where directed scientific research lead to new products, and the first where this occurred regularly.





















