Brass is an alloy of copper (Cu) and zinc (Zn), in proportions which can be varied to achieve varying mechanical, electrical, and chemical properties. It is a substitutional alloy: atoms of the two constituents may replace each other within the same crystal structure.

Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals and sometimes non-metals, such as phosphorus, or metalloids such as arsenic, or silicon. These additions produce a range of alloys that may be harder than copper alone, or have other useful properties, such as strength, ductility, or machinability.

Jewellery or jewelry consists of decorative items worn for personal adornment, such as brooches, rings, necklaces, earrings, pendants, bracelets, and cufflinks. Jewellery may be attached to the body or the clothes. From a western perspective, the term is restricted to durable ornaments, excluding flowers for example. For many centuries metal such as gold often combined with gemstones, has been the normal material for jewellery, but other materials such as shells and other plant materials may be used.
Pewter is a malleable metal alloy consisting of tin (85–99%), antimony, copper (2%), bismuth, and sometimes silver. Copper and antimony act as hardeners but lead may be used in lower grades of pewter, imparting a bluish tint. Pewter has a low melting point, around 170–230 °C (338–446 °F), depending on the exact mixture of metals. The word pewter is probably a variation of the word spelter, a term for zinc alloys.

Gilding metal is a form of brass with a much higher copper content than zinc content. Exact figures range from 95% copper and 5% zinc to “8 parts copper to 1 of zinc” in British Army Dress Regulations.
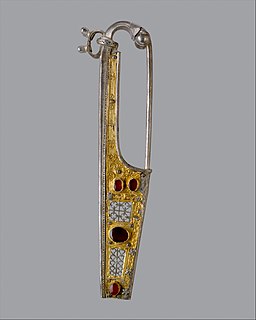
A brooch is a decorative jewelry item designed to be attached to garments, often to fasten them together. It is usually made of metal, often silver or gold or some other material. Brooches are frequently decorated with enamel or with gemstones and may be solely for ornament or serve a practical function as a clothes fastener. The earliest known brooches are from the Bronze Age. As fashions in brooches changed rather quickly, they are important chronological indicators. In archaeology, ancient European brooches are usually referred to by the Latin term fibula.

Orichalcum or aurichalcum is a metal mentioned in several ancient writings, including the story of Atlantis in the Critias of Plato. Within the dialogue, Critias claims that orichalcum had been considered second only to gold in value and had been found and mined in many parts of Atlantis in ancient times, but that by Critias's own time orichalcum was known only by name.
The year 1720 in science and technology involved some significant events.
In modern Western body piercing, a wide variety of materials are used. Some cannot be autoclaved, and others may induce allergic reactions, or harbour bacteria. Certain countries, such as those belonging to the EU, have legal regulations specifying which materials can be used in new piercings.
Plating is a surface covering in which a metal is deposited on a conductive surface. Plating has been done for hundreds of years; it is also critical for modern technology. Plating is used to decorate objects, for corrosion inhibition, to improve solderability, to harden, to improve wearability, to reduce friction, to improve paint adhesion, to alter conductivity, to improve IR reflectivity, for radiation shielding, and for other purposes. Jewelry typically uses plating to give a silver or gold finish.
The fineness of a precious metal object represents the weight of fine metal therein, in proportion to the total weight which includes alloying base metals and any impurities. Alloy metals are added to increase hardness and durability of coins and jewelry, alter colors, decrease the cost per weight, or avoid the cost of high-purity refinement. For example, copper is added to the precious metal silver to make a more durable alloy for use in coins, housewares and jewelry. Coin silver, which was used for making silver coins in the past, contains 90% silver and 10% copper, by mass. Sterling silver contains 92.5% silver and 7.5% of other metals, usually copper, by mass.

Metal clay is a crafting medium consisting of very small particles of metal such as silver, gold, bronze, or copper mixed with an organic binder and water for use in making jewelry, beads and small sculptures. Originating in Japan in 1990, metal clay can be shaped just like any soft clay, by hand or using molds. After drying, the clay can be fired in a variety of ways such as in a kiln, with a handheld gas torch, or on a gas stove, depending on the type of clay and the metal in it. The binder burns away, leaving the pure sintered metal. Shrinkage of between 8% and 30% occurs. Alloys such as bronze, sterling silver, and steel also are available.

Panchaloha, also called Pañcadhātu, is a term for traditional five-metal alloys of sacred significance, used for making Hindu temple murti and jewelry.

Wire sculpture is the creation of sculpture or jewelry out of wire. The use of metal wire in jewelry dates back to the 2nd Dynasty in Egypt and to the Bronze and Iron Ages in Europe. In the 20th century, the works of Alexander Calder, Ruth Asawa, and other modern practitioners developed the medium of wire sculpture as an art form.

Pure gold is slightly reddish yellow in colour, but coloured gold in various other colours can be produced by alloying gold with other elements.

Group 11, by modern IUPAC numbering, is a group of chemical elements in the periodic table, consisting of copper (Cu), silver (Ag), and gold (Au). Roentgenium (Rg) is also placed in this group in the periodic table, although no chemical experiments have yet been carried out to confirm that it behaves like the heavier homologue to gold. Group 11 is also known as the coinage metals, due to their usage in minting coins—while the rise in metal prices mean that silver and gold are no longer used for circulating currency, remaining in use for bullion, copper remains a common metal in coins to date, either in the form of copper clad coinage or as part of the cupronickel alloy. They were most likely the first three elements discovered. Copper, silver, and gold all occur naturally in elemental form.

The BIS hallmark is a hallmarking system for gold as well as silver jewellery sold in India certifying the purity of the metal. It certifies that the piece of jewellery conforms to a set of standards laid by the Bureau of Indian Standards, the national standards organization of India. India is the second biggest market for gold and its jewellery.

Traditional metal working in Mexico dates from the Mesoamerican period with metals such as gold, silver and copper. Other metals were mined and worked starting in the colonial period. The working of gold and silver, especially for jewelry, initially declined after the Spanish conquest of the Aztec Empire. However, during the colonial period, the working of metals rose again and took on much of the character traditional goods still have. Today, important metal products include those from silver, gold, copper, iron, tin and more made into jewelry, household objects, furniture, pots, decorative objects, toys and more. Important metal working centers include Taxco for silver, Santa Clara del Cobre for copper, Celaya for tin and Zacatecas for wrought iron.

On 25 November 2019, royal jewellery was stolen from the Green Vault museum within Dresden Castle in Dresden, Saxony, Germany. The stolen items include the 49-carat Dresden White Diamond, the diamond-laden breast star of the Polish Order of the White Eagle which belonged to the King of Poland, a hat clasp with a 16-carat diamond, a diamond epaulette, and a diamond-studded hilt containing nine large and 770 smaller diamonds, along with a matching scabbard. The missing items were of great cultural value to the State of Saxony and were described as priceless; other sources estimate the total value at about €1 billion.














