
Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. They are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall sandwiched between an inner cytoplasmic cell membrane and a bacterial outer membrane.

Levofloxacin, sold under the brand name Levaquin among others, is an antibiotic medication. It is used to treat a number of bacterial infections including acute bacterial sinusitis, pneumonia, H. pylori, urinary tract infections, chronic prostatitis, and some types of gastroenteritis. Along with other antibiotics it may be used to treat tuberculosis, meningitis, or pelvic inflammatory disease. Use is generally recommended only when other options are not available. It is available by mouth, intravenously, and in eye drop form.

A broad-spectrum antibiotic is an antibiotic that acts on the two major bacterial groups, Gram-positive and Gram-negative, or any antibiotic that acts against a wide range of disease-causing bacteria. These medications are used when a bacterial infection is suspected but the group of bacteria is unknown or when infection with multiple groups of bacteria is suspected. This is in contrast to a narrow-spectrum antibiotic, which is effective against only a specific group of bacteria. Although powerful, broad-spectrum antibiotics pose specific risks, particularly the disruption of native, normal bacteria and the development of antimicrobial resistance. An example of a commonly used broad-spectrum antibiotic is ampicillin.

Campylobacteriosis is an infection by the Campylobacter bacterium, most commonly C. jejuni. It is among the most common bacterial infections of humans, often a foodborne illness. It produces an inflammatory, sometimes bloody, diarrhea or dysentery syndrome, mostly including cramps, fever and pain.
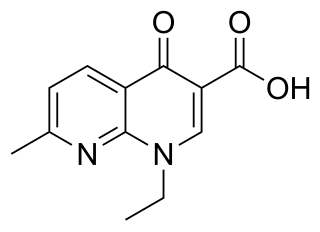
Nalidixic acid is the first of the synthetic quinolone antibiotics.
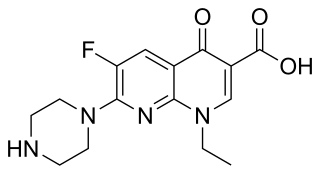
Enoxacin is an oral broad-spectrum fluoroquinolone antibacterial agent used in the treatment of urinary tract infections and gonorrhea. Insomnia is a common adverse effect. It is no longer available in the United States.
The Conrad–Limpach synthesis is the condensation of anilines (1) with β-ketoesters (2) to form 4-hydroxyquinolines (4) via a Schiff base (3). The overall reaction type is a combination of both an addition reaction as well as a rearrangement reaction. This reaction was discovered by Max Conrad (1848–1920) and Leonhard Limpach (1852–1933) in 1887 while they were studying the synthesis of quinoline derivatives.

Cinoxacin is a quinolone antibiotic that has been discontinued in the U.K. as well the United States, both as a branded drug or a generic. The marketing authorization of cinoxacin has been suspended throughout the EU.
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular reproduction and DNA organization, as they mediate the cleavage of single and double stranded DNA to relax supercoils, untangle catenanes, and condense chromosomes in eukaryotic cells. Topoisomerase inhibitors influence these essential cellular processes. Some topoisomerase inhibitors prevent topoisomerases from performing DNA strand breaks while others, deemed topoisomerase poisons, associate with topoisomerase-DNA complexes and prevent the re-ligation step of the topoisomerase mechanism. These topoisomerase-DNA-inhibitor complexes are cytotoxic agents, as the un-repaired single- and double stranded DNA breaks they cause can lead to apoptosis and cell death. Because of this ability to induce apoptosis, topoisomerase inhibitors have gained interest as therapeutics against infectious and cancerous cells.

Bartonella bacilliformis is a bacterium, Gram negative aerobic, pleomorphic, flagellated, motile, coccobacillary, 2–3 μm long, 0.2–0.5 μm wide, and a facultative intracellular bacterium.
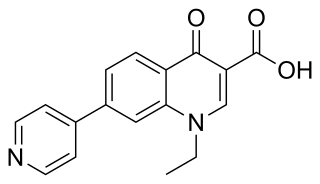
Rosoxacin is a quinolone antibiotic indicated for the treatment of urinary tract infections and certain sexually transmitted diseases. Rosoxacin is not available in the United States.
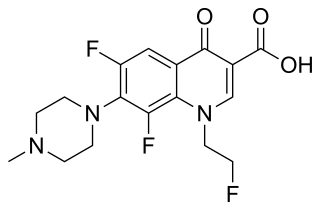
Fleroxacin is a quinolone antibiotic. It is sold under the brand names Quinodis and Megalocin.

Flumequine is a synthetic fluoroquinolone antibiotic used to treat bacterial infections. It is a first-generation fluoroquinolone antibacterial that has been removed from clinical use and is no longer being marketed. The marketing authorization of flumequine has been suspended throughout the EU. It kills bacteria by interfering with the enzymes that cause DNA to unwind and duplicate. Flumequine was used in veterinarian medicine for the treatment of enteric infections, as well as to treat cattle, swine, chickens, and fish, but only in a limited number of countries. It was occasionally used in France to treat urinary tract infections under the trade name Apurone. However this was a limited indication because only minimal serum levels were achieved.

Prulifloxacin is an older synthetic antibiotic of the fluoroquinolone class undergoing clinical trials prior to a possible NDA submission to the U.S. Food and Drug Administration (FDA). It is a prodrug which is metabolized in the body to the active compound ulifloxacin. It was developed over two decades ago by Nippon Shinyaku Co. and was patented in Japan in 1987 and in the United States in 1989.

Amfonelic acid is a research chemical and dopaminergic stimulant with antibiotic properties.

SER-601 (COR-167) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist, based on a quinolone-3-carboxylic acid core structure, with 190 times selectivity for CB2 over the related CB1 receptor. It has analgesic effects in animal studies, as well as neuroprotective effects, but without a "cannabis high" due to its low affinity for CB1. A number of related compounds are known, almost all of which have high selectivity for CB2.

Quinolone antibiotics constitute a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone. They are used in human and veterinary medicine to treat bacterial infections, as well as in animal husbandry, specifically poultry production.

Nemonoxacin is a non-fluorinated quinolone antibiotic undergoing clinical trials. It has the same mechanism of action as fluouroquinolones; it inhibits DNA gyrase, preventing DNA synthesis, gene duplication, and cell division. At the end of 2016, it had reached market in Taiwan, Russia, the Commonwealth Independent States, Turkey, mainland China, and Latin America under the brand name Taigexyn. Nemonoxacin has completed phase 2 trials in the US and has moved on to phase 3 trials. The U.S. Food and Drug Administration (FDA) has granted nemonoxacin qualified infectious disease product (QIDP) and fast track designations for community-acquired bacterial pneumonia (CAP) and acute bacterial skin and skin-structure infections (ABSSSI).
Ozenoxacin, sold under the brand names Ozanex and Xepi, is a quinolone antibiotic used for the treatment of impetigo. A 1% topical cream is approved for treatment of impetigo in Canada and in the United States.

Finafloxacin (Xtoro) is a fluoroquinolone antibiotic. In the United States, it is approved by the Food and Drug Administration to treat acute otitis externa caused by the bacteria Pseudomonas aeruginosa and Staphylococcus aureus.


















