Dicer, also known as endoribonuclease Dicer or helicase with RNase motif, is an enzyme that in humans is encoded by the DICER1 gene. Being part of the RNase III family, Dicer cleaves double-stranded RNA (dsRNA) and pre-microRNA (pre-miRNA) into short double-stranded RNA fragments called small interfering RNA and microRNA, respectively. These fragments are approximately 20–25 base pairs long with a two-base overhang on the 3′-end. Dicer facilitates the activation of the RNA-induced silencing complex (RISC), which is essential for RNA interference. RISC has a catalytic component Argonaute, which is an endonuclease capable of degrading messenger RNA (mRNA).
The RNA-induced silencing complex, or RISC, is a multiprotein complex, specifically a ribonucleoprotein, which functions in gene silencing via a variety of pathways at the transcriptional and translational levels. Using single-stranded RNA (ssRNA) fragments, such as microRNA (miRNA), or double-stranded small interfering RNA (siRNA), the complex functions as a key tool in gene regulation. The single strand of RNA acts as a template for RISC to recognize complementary messenger RNA (mRNA) transcript. Once found, one of the proteins in RISC, Argonaute, activates and cleaves the mRNA. This process is called RNA interference (RNAi) and it is found in many eukaryotes; it is a key process in defense against viral infections, as it is triggered by the presence of double-stranded RNA (dsRNA).

The Argonaute protein family, first discovered for its evolutionarily conserved stem cell function, plays a central role in RNA silencing processes as essential components of the RNA-induced silencing complex (RISC). RISC is responsible for the gene silencing phenomenon known as RNA interference (RNAi). Argonaute proteins bind different classes of small non-coding RNAs, including microRNAs (miRNAs), small interfering RNAs (siRNAs) and Piwi-interacting RNAs (piRNAs). Small RNAs guide Argonaute proteins to their specific targets through sequence complementarity, which then leads to mRNA cleavage, translation inhibition, and/or the initiation of mRNA decay.
Piwi-interacting RNA (piRNA) is the largest class of small non-coding RNA molecules expressed in animal cells. piRNAs form RNA-protein complexes through interactions with piwi-subfamily Argonaute proteins. These piRNA complexes are mostly involved in the epigenetic and post-transcriptional silencing of transposable elements and other spurious or repeat-derived transcripts, but can also be involved in the regulation of other genetic elements in germ line cells.
RNA-induced transcriptional silencing (RITS) is a form of RNA interference by which short RNA molecules – such as small interfering RNA (siRNA) – trigger the downregulation of transcription of a particular gene or genomic region. This is usually accomplished by posttranslational modification of histone tails which target the genomic region for heterochromatin formation. The protein complex that binds to siRNAs and interacts with the methylated lysine 9 residue of histones H3 (H3K9me2) is the RITS complex.
RNA silencing or RNA interference refers to a family of gene silencing effects by which gene expression is negatively regulated by non-coding RNAs such as microRNAs. RNA silencing may also be defined as sequence-specific regulation of gene expression triggered by double-stranded RNA (dsRNA). RNA silencing mechanisms are conserved among most eukaryotes. The most common and well-studied example is RNA interference (RNAi), in which endogenously expressed microRNA (miRNA) or exogenously derived small interfering RNA (siRNA) induces the degradation of complementary messenger RNA. Other classes of small RNA have been identified, including piwi-interacting RNA (piRNA) and its subspecies repeat associated small interfering RNA (rasiRNA).
oskar is a gene required for the development of the Drosophila embryo. It defines the posterior pole during early embryogenesis. Its two isoforms, short and long, play different roles in Drosophila embryonic development. oskar was named after the main character from the Günter Grass novel The Tin Drum, who refuses to grow up.

Protein argonaute-2 is a protein that in humans is encoded by the EIF2C2 gene.

Protein argonaute-1 is a protein that in humans is encoded by the EIF2C1 gene.

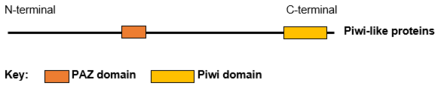
Piwi-like protein 1 is a protein that in humans is encoded by the PIWIL1 gene.

In molecular biology, a Tudor domain is a conserved protein structural domain originally identified in the Tudor protein encoded in Drosophila. The Tudor gene was found in a Drosophila screen for maternal factors that regulate embryonic development or fertility. Mutations here are lethal for offspring, inspiring the name Tudor, as a reference to the Tudor King Henry VIII and the several miscarriages experienced by his wives.
Nuage are Drosophila melanogaster germline granules. Nuage are the hallmark of Drosophila melanogaster germline cells, which have an electron-dense perinuclear structure and can silence the selfish genetic elements in Drosophila melanogaster. The term 'Nuage' comes from the French word for 'cloud', as they appear as nebulous electron-dense bodies by electron microscopy. They are found in nurse cells of the developing Drosophila melanogaster egg chamber and are composed of various types of proteins, including RNA-helicases, Tudor domain proteins, Piwi-clade Argonaute proteins, in addition to a PRMT5 methylosome composed of Capsuléen and its co-factor, Valois (MEP50).
Vasa is an RNA binding protein with an ATP-dependent RNA helicase that is a member of the DEAD box family of proteins. The vasa gene is essential for germ cell development and was first identified in Drosophila melanogaster, but has since been found to be conserved in a variety of vertebrates and invertebrates including humans. The Vasa protein is found primarily in germ cells in embryos and adults, where it is involved in germ cell determination and function, as well as in multipotent stem cells, where its exact function is unknown.

In molecular biology, bantam microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
Transposon silencing is a form of transcriptional gene silencing targeting transposons. Transcriptional gene silencing is a product of histone modifications that prevent the transcription of a particular area of DNA. Transcriptional silencing of transposons is crucial to the maintenance of a genome. The “jumping” of transposons generates genomic instability and can cause extremely deleterious mutations. Transposable element insertions have been linked to many diseases including hemophilia, severe combined immunodeficiency, and predisposition to cancer. The silencing of transposons is therefore extremely critical in the germline in order to stop transposon mutations from developing and being passed on to the next generation. Additionally, these epigenetic defenses against transposons can be heritable. Studies in Drosophila, Arabidopsis thaliana, and mice all indicate that small interfering RNAs are responsible for transposon silencing. In animals, these siRNAS and piRNAs are most active in the gonads.
RDE-1 (RNAi-DEfective 1) is a primary Argonaute protein required for RNA-mediated interference (RNAi) in Caenorhabditis elegans. The rde-1 gene locus was first characterized in C. elegans mutants resistant to RNAi, and is a member of a highly conserved Piwi gene family that includes plant, Drosophila, and vertebrate homologs.
The gene Maelstrom, Mael, creates a protein, which was first located in Drosophila melanogaster in the nuage perinuclear structure and has functionality analogous to the spindle, spn, gene class. Its mammalian homolog is MAEL.

Piwi like RNA-mediated gene silencing 4 is a protein that in humans is encoded by the PIWIL4 gene.
piwi-interacting RNA (piRNA) belongs to the small RNA class found in eukaryotic organisms, their major role is to regulate the expression of genes by the mRNA degradation or silencing. After the association with argonaute protein family, these short RNA guide the RNA-induced silencing complex (RISC) to their target by sequence complementarity. piRNAs have approximately from 26 to 31 nucleotides and are found in almost all metazoans.
Haifan Lin is a Chinese-born American stem cell biologist. He is the Eugene Higgins Chair Professor of Cell Biology at Yale University and the founding Director of the Yale Stem Cell Center. He previously founded and directed the Stem Cell Research Program at Duke University. Recognized for his significant contributions to stem cell research, he was elected to the US National Academy of Sciences and American Academy of Arts and Sciences in 2018.