Related Research Articles

Ethers are a class of organic compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R–O–R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (CH3–CH2–O–CH2–CH3). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

A thermoplastic, or thermosoft plastic, is a plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.

Acrylonitrile butadiene styrene (ABS) (chemical formula (C8H8)x·(C4H6)y·(C3H3N)z) is a common thermoplastic polymer. Its glass transition temperature is approximately 200 °C (392 °F). ABS is amorphous and therefore has no true melting point.

Polyether ether ketone (PEEK) is a colourless organic thermoplastic polymer in the polyaryletherketone (PAEK) family, used in engineering applications. The polymer was first developed in November 1978, later being introduced to the market by Victrex PLC, then Imperial Chemical Industries (ICI) in the early 1980s.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure, or mixing with a catalyst. Heat is not necessarily applied externally, but is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.

Polyimide is a polymer containing imide groups belonging to the class of high performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after chemist Carl Mannich.

Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.
Polysulfones are a family of high performance thermoplastics. These polymers are known for their toughness and stability at high temperatures. Technically used polysulfones contain an aryl-SO2-aryl subunit. Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

The Dakin oxidation is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde or ketone reacts with hydrogen peroxide in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidized, and the hydrogen peroxide is reduced.
The NORYL family of modified resins consists of amorphous blends of polyphenylene oxides (PPO) or polyphenylene ether (PPE) resins with polystyrene. They combine the inherent benefits of PPE resin, with excellent dimensional stability, good processability and low density.
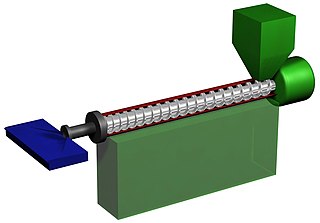
Plastics extrusion is a high-volume manufacturing process in which raw plastic is melted and formed into a continuous profile. Extrusion produces items such as pipe/tubing, weatherstripping, fencing, deck railings, window frames, plastic films and sheeting, thermoplastic coatings, and wire insulation.
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis.

Filler materials are particles added to resin or binders that can improve specific properties, make the product cheaper, or a mixture of both. The two largest segments for filler material use is elastomers and plastics. Worldwide, more than 53 million tons of fillers are used every year in application areas such as paper, plastics, rubber, paints, coatings, adhesives, and sealants. As such, fillers, produced by more than 700 companies, rank among the world's major raw materials and are contained in a variety of goods for daily consumer needs. The top filler materials used are ground calcium carbonate (GCC), precipitated calcium carbonate (PCC), kaolin, talc, and carbon black. Filler materials can affect the tensile strength, toughness, heat resistance, color, clarity etc. A good example of this is the addition of talc to polypropylene. Most of the filler materials used in plastics are mineral or glass based filler materials. Particulates and fibers are the main subgroups of filler materials. Particulates are small particles of filler which are mixed in the matrix where size and aspect ratio are important. Fibers are small circular strands that can be very long and have very high aspect ratios.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the space shuttle.
Carbonyl oxidation with hypervalent iodine reagents involves the functionalization of the α position of carbonyl compounds through the intermediacy of a hypervalent iodine(III) enolate species. This electrophilic intermediate may be attacked by a variety of nucleophiles or undergo rearrangement or elimination.
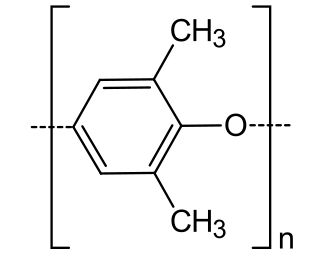
Poly(p-phenylene oxide) or poly(p-phenylene ether) (PPE) is a high-temperature thermoplastic. It is rarely used in its pure form due to difficulties in processing. It is mainly used as blend with polystyrene, high impact styrene-butadiene copolymer or polyamide. PPO is a registered trademark of SABIC Innovative Plastics IP B.V. under which various polyphenylene ether resins are sold.

High-performance plastics are plastics that meet higher requirements than standard or engineering plastics. They are more expensive and used in smaller amounts.
References
- 1 2 3 Salamone 1999 , p. 1102.
- 1 2 Rosato & Rosato 2004 , p. 81.
- 1 2 3 Rosato & Rosato 2004 , p. 82.
- 1 2 3 Salamone 1996 , p. 5548.
- ↑ Salamone 1996 , p. 5549.
Bibliography
- Rosato, Dominick V.; Rosato, Donald V. (2004), Plastic product material and process selection handbook, Elsevier, ISBN 978-1-85617-431-2 .
- Salamone, Joseph C. (1999), Concise polymeric materials encyclopedia, CRC Press, ISBN 978-0-8493-2226-6 .
- Salamone, Joseph C. (1996), Salamone, Joseph C. (ed.), Polymeric materials encyclopedia, vol. 4, CRC Press, ISBN 978-0-8493-2470-3 .