
Phenol is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (−C6H5) bonded to a hydroxy group (−OH). Mildly acidic, it requires careful handling because it can cause chemical burns.

A polychlorinated biphenyl (PCB) is an organic chlorine compound with the formula C12H10−xClx. Polychlorinated biphenyls were once widely deployed as dielectric and coolant fluids in electrical apparatus, carbonless copy paper and in heat transfer fluids.
Polychlorinated dibenzodioxins (PCDDs), or simply dioxins, are a group of polyhalogenated organic compounds that are significant environmental pollutants.
Triclosan is an antibacterial and antifungal agent present in some consumer products, including toothpaste, soaps, detergents, toys, and surgical cleaning treatments. It is similar in its uses and mechanism of action to triclocarban. Its efficacy as an antimicrobial agent, the risk of antimicrobial resistance, and its possible role in disrupted hormonal development remains controversial. Additional research seeks to understand its potential effects on organisms and environmental health.
Polybrominated diphenyl ethers or PBDEs, are organobromine compounds that are used as flame retardant. Like other brominated flame retardants, PBDEs have been used in a wide array of products, including building materials, electronics, furnishings, motor vehicles, airplanes, plastics, polyurethane foams, and textiles. They are structurally akin to the polychlorinated biphenyls (PCBs) and other polyhalogenated compounds, consisting of two halogenated aromatic rings. PBDEs are classified according to the average number of bromine atoms in the molecule. The health hazards of these chemicals have attracted increasing scrutiny, and they have been shown to reduce fertility in humans at levels found in households. Their chlorine analogs are polychlorinated diphenyl ethers (PCDEs). Because of their toxicity and persistence, the industrial production of some PBDEs is restricted under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POPs).
The term flame retardants subsumes a diverse group of chemicals which are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source and are intended to prevent or slow the further development of ignition by a variety of different physical and chemical methods. They may be added as a copolymer during the polymerisation process, or later added to the polymer at a moulding or extrusion process or applied as a topical finish. Mineral flame retardants are typically additive while organohalogen and organophosphorus compounds can be either reactive or additive.
Reductive dechlorination is degradation of chlorinated organic compounds by chemical reduction with release of inorganic chloride ions by reductive dehalogenases.
Persistent organic pollutants (POPs) are organic compounds that are resistant to environmental degradation through chemical, biological, and photolytic processes. Because of their persistence, POPs bioaccumulate with potential adverse impacts on human health and the environment. The effect of POPs on human and environmental health was discussed, with intention to eliminate or severely restrict their production, by the international community at the Stockholm Convention on Persistent Organic Pollutants in 2001.

Hand sanitizer is a liquid generally used to decrease infectious agents on the hands. Formulations of the alcohol-based type are preferable to hand washing with soap and water in most situations in the healthcare setting. It is generally more effective at killing microorganisms and better tolerated than soap and water. Hand washing should still be carried out if contamination can be seen or following the use of the toilet. The general use of non-alcohol based versions has no recommendations. Outside the health care setting evidence to support the use of hand sanitizer over hand washing is poor. They are available as liquids, gels, and foams.
Dehalococcoides is a genus of bacteria within class Dehalococcoidia that obtain energy via the oxidation of hydrogen and subsequent reductive dehalogenation of halogenated organic compounds in a mode of anaerobic respiration called organohalide respiration. They are well known for their great potential to remediate halogenated ethenes and aromatics. They are the only bacteria known to transform highly chlorinated dioxins, PCBs. In addition, they are the only known bacteria to transform tetrachloroethene to ethene.

Triclocarban is an antibacterial chemical once common in, but now phased out of, personal care products like soaps and lotions. It was originally developed for the medical field. Although the mode of action is unknown, TCC can be effective in fighting infections by targeting the growth of bacteria such as Staphylococcus aureus. Additional research seeks to understand its potential for causing antibacterial resistance and its effects on organismal and environmental health.

1,4-Dioxin (also referred as dioxin or p-dioxin) is a heterocyclic, organic, non-aromatic compound with the chemical formula C4H4O2. There is an isomeric form of 1,4-dioxin, 1,2-dioxin (or o-dioxin). 1,2-Dioxin is very unstable due to its peroxide-like characteristics.

Polychlorinated dibenzofurans (PCDFs) are a family of organic compounds with one or several of the hydrogens in the dibenzofuran structure replaced by chlorines. For example, 2,3,7,8-tetrachlorodibenzofuran (TCDF) has chlorine atoms substituted for each of the hydrogens on the number 2, 3, 7, and 8 carbons. Polychlorinated dibenzofurans are much more toxic than the parent compounds, with properties and chemical structures similar to polychlorinated dibenzodioxins. These groups together are often inaccurately called dioxins. They are known teratogens, mutagens, and suspected human carcinogens. PCDFs tend to co-occur with polychlorinated dibenzodioxins (PCDDs). PCDFs can be formed by pyrolysis or incineration at temperatures below 1200 °C of chlorine containing products, such as PVC, PCBs, and other organochlorides, or of non-chlorine containing products in the presence of chlorine donors. Dibenzofurans are known persistent organic pollutants (POP), classified among the dirty dozen in the Stockholm Convention on Persistent Organic Pollutants.
2,4-Dichlorophenol (2,4-DCP) is a chlorinated derivative of phenol with the molecular formula Cl2C6H3OH. It is a white solid that is mildly acidic (pKa = 7.9). It is produced on a large scale as a precursor to the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D).

Dioxins and dioxin-like compounds (DLCs) are compounds that are highly toxic environmental persistent organic pollutants (POPs). They are mostly by-products of various industrial processes - or, in case of dioxin-like PCBs and PBBs, part of intentionally produced mixtures. They include:
Polyhalogenated compounds (PHCs) are any compounds with multiple substitutions of halogens. They are of particular interest and importance because they bioaccumulate in humans, and comprise a superset of which has many toxic and carcinogenic industrial chemicals as members. PBDEs, PCBs, dioxins (PCDDs) and PFCs are all polyhalogenated compounds. They are generally non-miscible in organic solvents or water, but miscible in some hydrocarbons from which they often derive.
Chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs) are a group of compounds comprising polycyclic aromatic hydrocarbons with two or more aromatic rings and one or more chlorine atoms attached to the ring system. Cl-PAHs can be divided into two groups: chloro-substituted PAHs, which have one or more hydrogen atoms substituted by a chlorine atom, and chloro-added Cl-PAHs, which have two or more chlorine atoms added to the molecule. They are products of incomplete combustion of organic materials. They have many congeners, and the occurrences and toxicities of the congeners differ. Cl-PAHs are hydrophobic compounds and their persistence within ecosystems is due to their low water solubility. They are structurally similar to other halogenated hydrocarbons such as polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and polychlorinated biphenyls (PCBs). Cl-PAHs in the environment are strongly susceptible to the effects of gas/particle partitioning, seasonal sources, and climatic conditions.

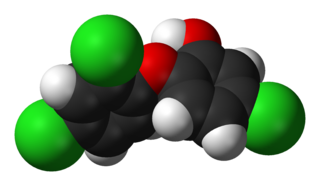
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a polychlorinated dibenzo-p-dioxin with the chemical formula C
12H
4Cl
4O
2. Pure TCDD is a colorless solid with no distinguishable odor at room temperature. It is usually formed as a side product in organic synthesis and burning of organic materials.
Adsorbable Organic Halides (AOX) is a measure of the organic halogen load at a sampling site such as soil from a land fill, water, or sewage waste. The procedure measures chlorine, bromine, and iodine as equivalent halogens, but does not measure fluorine levels in the sample.

1,2,3,4,6,7,8-Heptachlorodibenzo-para-dioxin (often referred to as 1,2,3,4,6,7,8-HpCDD) is a polychlorinated derivative of dibenzo-p-dioxin and can therefore be categorized as polychlorinated dibenzo-p-dioxin (PCDD), a subclass of dioxins which includes 75 congeners. HpCDD is the dibenzo-p-dioxin which is chlorinated at positions 1, 2, 3, 4, 6, 7, and 8. It is a polycyclic heterocyclic organic compound, since HpCDD contains multiple cyclic structures (two benzene rings connected by a 1,4-dioxin ring) in which two different elements (carbon and oxygen) are members of its rings. HpCDD has molecular formula C12HCl7O2 and is an off-white powder, which is insoluble in water.









