Related Research Articles

Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.

Autosomal dominant polycystic kidney disease (ADPKD) is one of the most common, life-threatening inherited human disorders and the most common hereditary kidney disease. It is associated with large interfamilial and intrafamilial variability, which can be explained to a large extent by its genetic heterogeneity and modifier genes. It is also the most common of the inherited cystic kidney diseases — a group of disorders with related but distinct pathogenesis, characterized by the development of renal cysts and various extrarenal manifestations, which in case of ADPKD include cysts in other organs, such as the liver, seminal vesicles, pancreas, and arachnoid membrane, as well as other abnormalities, such as intracranial aneurysms and dolichoectasias, aortic root dilatation and aneurysms, mitral valve prolapse, and abdominal wall hernias. Over 50% of patients with ADPKD eventually develop end stage kidney disease and require dialysis or kidney transplantation. ADPKD is estimated to affect at least one in every 1000 individuals worldwide, making this disease the most common inherited kidney disorder with a diagnosed prevalence of 1:2000 and incidence of 1:3000-1:8000 in a global scale.
Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN, and TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of tastes, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.

Chloride channels are a superfamily of poorly understood ion channels specific for chloride. These channels may conduct many different ions, but are named for chloride because its concentration in vivo is much higher than other anions. Several families of voltage-gated channels and ligand-gated channels have been characterized in humans.
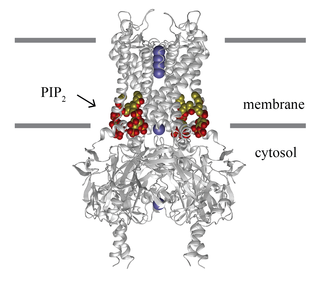
Inward-rectifier potassium channels (Kir, IRK) are a specific lipid-gated subset of potassium channels. To date, seven subfamilies have been identified in various mammalian cell types, plants, and bacteria. They are activated by phosphatidylinositol 4,5-bisphosphate (PIP2). The malfunction of the channels has been implicated in several diseases. IRK channels possess a pore domain, homologous to that of voltage-gated ion channels, and flanking transmembrane segments (TMSs). They may exist in the membrane as homo- or heterooligomers and each monomer possesses between 2 and 4 TMSs. In terms of function, these proteins transport potassium (K+), with a greater tendency for K+ uptake than K+ export. The process of inward-rectification was discovered by Denis Noble in cardiac muscle cells in 1960s and by Richard Adrian and Alan Hodgkin in 1970 in skeletal muscle cells.

Fibrocystin is a large, receptor-like protein that is thought to be involved in the tubulogenesis and/or maintenance of duct-lumen architecture of epithelium. FPC associates with the primary cilia of epithelial cells and co-localizes with the Pkd2 gene product polycystin-2 (PC2), suggesting that these two proteins may function in a common molecular pathway.
TRPML comprises a group of three evolutionarily related proteins that belongs to the large family of transient receptor potential ion channels. The three proteins TRPML1, TRPML2 and TRPML3 are encoded by the mucolipin-1 (MCOLN1), mucolipin-2 (MCOLN2) and mucolipin-3 (MCOLN3) genes, respectively.
TRPP is a family of transient receptor potential ion channels which when mutated can cause polycystic kidney disease.

Polycystin 1 (PC1) is a protein that in humans is encoded by the PKD1 gene. Mutations of PKD1 are associated with most cases of autosomal dominant polycystic kidney disease, a severe hereditary disorder of the kidneys characterised by the development of renal cysts and severe kidney dysfunction.

Transient receptor potential canonical 1 (TRPC1) is a protein that in humans is encoded by the TRPC1 gene.

Polycystin-2(PC2) is a protein that in humans is encoded by the PKD2 gene.
In molecular biology, zinc-dependent phospholipases C is a family of bacterial phospholipases C enzymes, some of which are also known as alpha toxins.

Polycystic kidney disease 2-like 1 protein also known as transient receptor potential polycystic 2 is a protein that in humans is encoded by the PKD2L1 gene.

Polycystic kidney disease is a genetic disorder in which the renal tubules become structurally abnormal, resulting in the development and growth of multiple cysts within the kidney. These cysts may begin to develop in utero, in infancy, in childhood, or in adulthood. Cysts are non-functioning tubules filled with fluid pumped into them, which range in size from microscopic to enormous, crushing adjacent normal tubules and eventually rendering them non-functional as well.

Autosomal recessive polycystic kidney disease (ARPKD) is the recessive form of polycystic kidney disease. It is associated with a group of congenital fibrocystic syndromes. Mutations in the PKHD1 cause ARPKD.

Polycystic kidney disease 2-like 2 protein (PKD2L2) also known as transient receptor potential polycystic 5 (TRPP5) is a protein that in humans is encoded by the PKD2L2 gene.
PKD domain was first identified in the polycystic kidney disease protein, polycystin-1, and contains an Ig-like fold consisting of a beta-sandwich of seven strands in two sheets with a Greek key topology, although some members have additional strands. Polycystin-1 is a large cell-surface glycoprotein involved in adhesive protein–protein and protein–carbohydrate interactions; however it is not clear if the PKD domain mediates any of these interactions.
The transient receptor potential Ca2+ channel (TRP-CC) family (TC# 1.A.4) is a member of the voltage-gated ion channel (VIC) superfamily and consists of cation channels conserved from worms to humans. The TRP-CC family also consists of seven subfamilies (TRPC, TRPV, TRPM, TRPN, TRPA, TRPP, and TRPML) based on their amino acid sequence homology:
- the canonical or classic TRPs,
- the vanilloid receptor TRPs,
- the melastatin or long TRPs,
- ankyrin (whose only member is the transmembrane protein 1 [TRPA1])
- TRPN after the nonmechanoreceptor potential C (nonpC), and the more distant cousins,
- the polycystins
- and mucolipins.
The K+Transporter (Trk) Family is a member of the voltage-gated ion channel (VIC) superfamily. The proteins of the Trk family are derived from Gram-negative and Gram-positive bacteria, yeast and plants.

Polycystic kidney disease 3 (autosomal dominant) is a protein that in humans is encoded by the PKD3 gene.
References
- ↑ "1.A.5 The Polycystin Cation Channel (PCC) Family". TCDB. Retrieved 10 April 2016.
- ↑ "P98161-PKD1 Human". Uniprot.
- ↑ Oatley P, Stewart AP, Sandford R, Edwardson JM (April 2012). "Atomic force microscopy imaging reveals the domain structure of polycystin-1". Biochemistry. 51 (13): 2879–88. doi:10.1021/bi300134b. PMID 22409330.
- ↑ Dalagiorgou G, Basdra EK, Papavassiliou AG (October 2010). "Polycystin-1: function as a mechanosensor". The International Journal of Biochemistry & Cell Biology. 42 (10): 1610–3. doi:10.1016/j.biocel.2010.06.017. PMID 20601082.
- ↑ Molland KL, Narayanan A, Burgner JW, Yernool DA (July 2010). "Identification of the structural motif responsible for trimeric assembly of the C-terminal regulatory domains of polycystin channels PKD2L1 and PKD2". The Biochemical Journal. 429 (1): 171–83. doi:10.1042/BJ20091843. PMID 20408813.
- ↑ Anyatonwu GI, Ehrlich BE (August 2005). "Organic cation permeation through the channel formed by polycystin-2". The Journal of Biological Chemistry. 280 (33): 29488–93. doi: 10.1074/jbc.M504359200 . PMID 15961385.
- ↑ Gonzalez-Perrett S, Batelli M, Kim K, Essafi M, Timpanaro G, Moltabetti N, Reisin IL, Arnaout MA, Cantiello HF (July 2002). "Voltage dependence and pH regulation of human polycystin-2-mediated cation channel activity". The Journal of Biological Chemistry. 277 (28): 24959–66. doi: 10.1074/jbc.M105084200 . PMID 11991947.
- ↑ Liu Y, Li Q, Tan M, Zhang YY, Karpinski E, Zhou J, Chen XZ (August 2002). "Modulation of the human polycystin-L channel by voltage and divalent cations". FEBS Letters. 525 (1–3): 71–6. doi: 10.1016/s0014-5793(02)03071-5 . PMID 12163164. S2CID 3150744.
- ↑ Xu GM, González-Perrett S, Essafi M, Timpanaro GA, Montalbetti N, Arnaout MA, Cantiello HF (January 2003). "Polycystin-1 activates and stabilizes the polycystin-2 channel". The Journal of Biological Chemistry. 278 (3): 1457–62. doi:10.1074/jbc.M209996200. PMID 12407099.
- ↑ Noben-Trauth K (1 January 2011). "The TRPML3 channel: from gene to function". Advances in Experimental Medicine and Biology. 704: 229–37. doi:10.1007/978-94-007-0265-3_13. ISBN 978-94-007-0264-6. PMID 21290299.
- 1 2 Li Q, Dai XQ, Shen PY, Wu Y, Long W, Chen CX, Hussain Z, Wang S, Chen XZ (December 2007). "Direct binding of alpha-actinin enhances TRPP3 channel activity". Journal of Neurochemistry. 103 (6): 2391–400. doi: 10.1111/j.1471-4159.2007.04940.x . PMID 17944866. S2CID 84357640.
- ↑ Kiselyov K, Chen J, Rbaibi Y, Oberdick D, Tjon-Kon-Sang S, Shcheynikov N, Muallem S, Soyombo A (December 2005). "TRP-ML1 is a lysosomal monovalent cation channel that undergoes proteolytic cleavage". The Journal of Biological Chemistry. 280 (52): 43218–23. doi: 10.1074/jbc.M508210200 . PMID 16257972.
- ↑ Kim I, Fu Y, Hui K, Moeckel G, Mai W, Li C, Liang D, Zhao P, Ma J, Chen XZ, George AL, Coffey RJ, Feng ZP, Wu G (March 2008). "Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function". Journal of the American Society of Nephrology. 19 (3): 455–68. doi:10.1681/ASN.2007070770. PMC 2391052 . PMID 18235088.
As of this edit, this article uses content from "1.A.5 The Polycystin Cation Channel (PCC) Family" , which is licensed in a way that permits reuse under the Creative Commons Attribution-ShareAlike 3.0 Unported License, but not under the GFDL. All relevant terms must be followed.